


Monitoring success in clinical trials hinges on three key practices:
These practices are crucial as they enhance the reliability and efficiency of trials. By ensuring adherence to regulatory guidelines, utilizing advanced technologies such as remote patient monitoring and electronic data collection, and fostering open communication alongside regular performance reviews among research teams, organizations can significantly improve trial outcomes. Embracing these practices not only addresses existing challenges but also positions clinical research for future advancements.
In the intricate world of clinical trials, the stakes are undeniably high. The success of groundbreaking therapies hinges on meticulous monitoring practices. Establishing robust compliance standards, embracing innovative technologies, and implementing effective strategies are essential for ensuring the integrity and reliability of research outcomes. However, as the regulatory landscape evolves and new methodologies emerge, organizations must navigate these complexities to enhance both safety and efficacy in their clinical studies. This article delves into three key practices that can transform monitoring efforts and ultimately lead to successful trial outcomes.
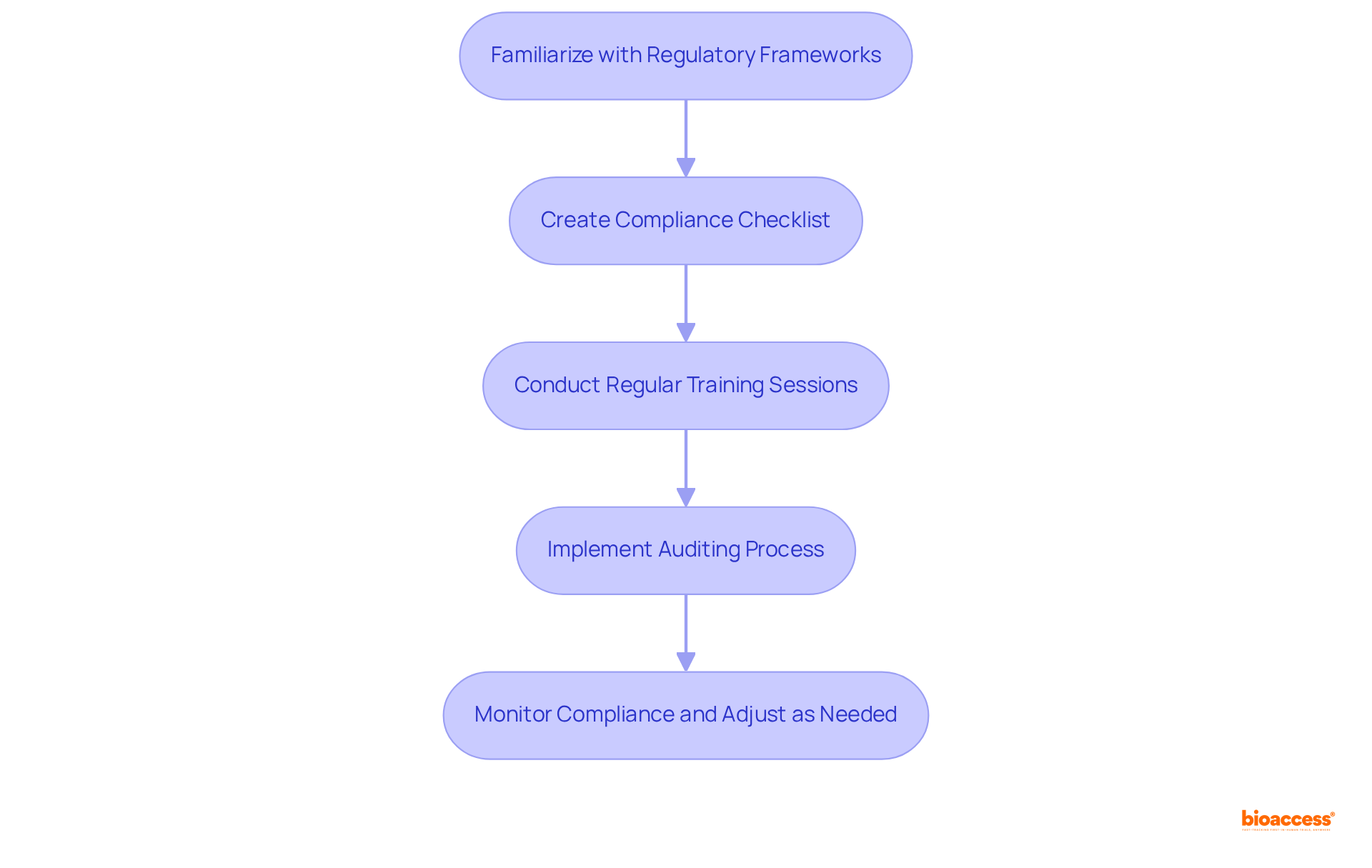
To establish compliance standards for monitoring in clinical trials, organizations must first familiarize themselves with the relevant regulatory frameworks, particularly the Good Clinical Practice (GCP) guidelines and local regulations. This critical process begins with the creation of a comprehensive compliance checklist that encompasses essential elements such as:
Regular training sessions for the research team are vital for effective monitoring in clinical trials, ensuring that all members understand their responsibilities and the importance of adhering to these standards, especially as the regulatory landscape evolves.
Furthermore, implementing a robust auditing process is essential for monitoring in clinical trials, as it helps to identify compliance gaps early and enables timely corrective actions. For instance, a study conducted by a notable biopharma firm demonstrated that regular compliance audits resulted in a 30% decrease in protocol deviations, significantly enhancing the study's integrity and reliability.
Non-compliance directly affects the validity and reliability of study results, rendering them inconclusive. As GCP guidelines continue to evolve, particularly with updates scheduled to begin in July 2025, remaining informed and proactive in compliance efforts will be essential for the success of research studies. Adherence not only boosts the credibility of experiments but also strengthens the scientific community's trust by enhancing the reproducibility of results.
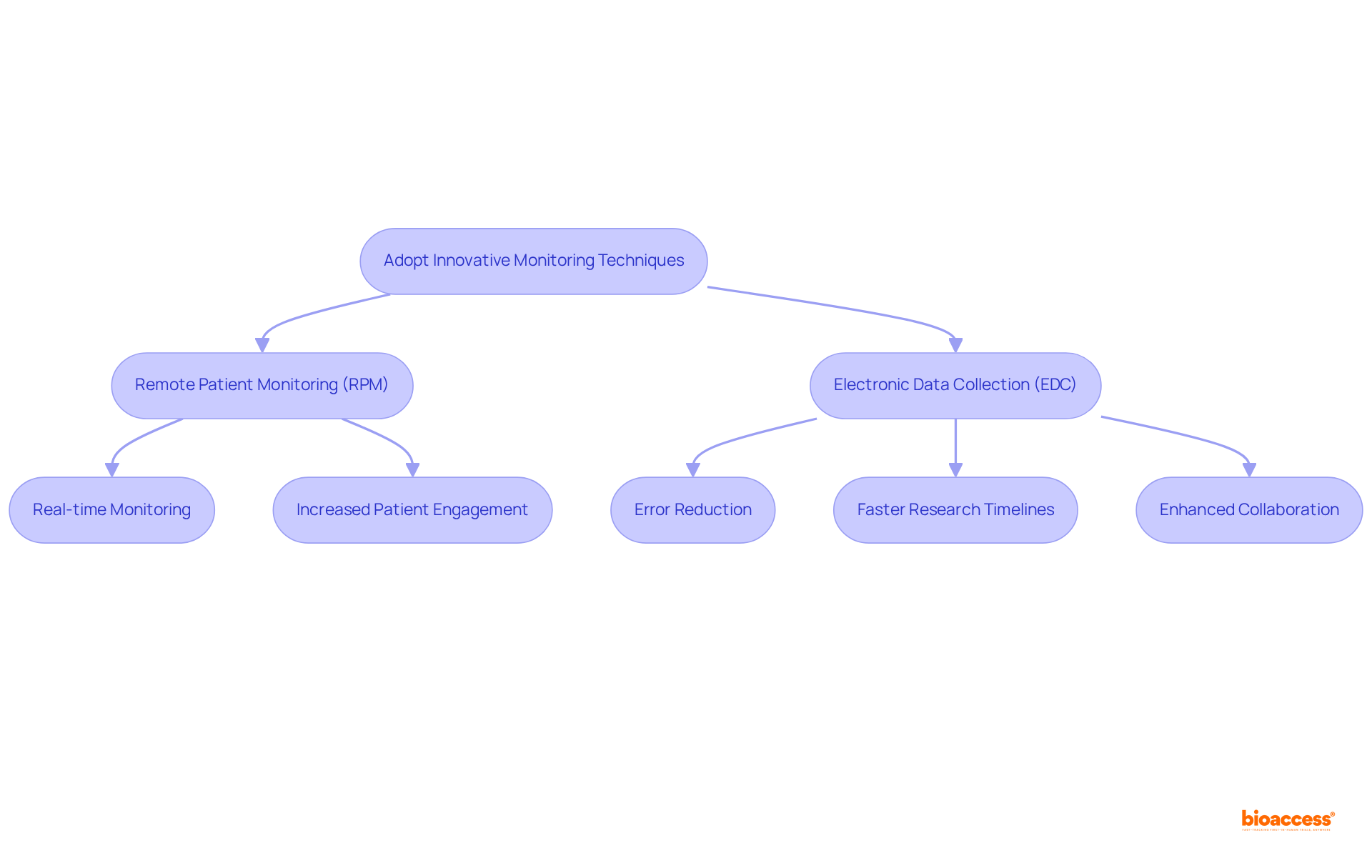
Applying innovative oversight methods such as remote patient observation (RPM) and electronic information collection (EDC) significantly enhances monitoring in clinical trials for safety and effectiveness. RPM facilitates ongoing information gathering from patients beyond traditional clinical settings, allowing for real-time monitoring of vital signs and symptoms. This approach not only increases patient engagement but also supports effective monitoring in clinical trials to ensure timely responses to adverse events.
For example, a recent cardiovascular study that utilized RPM achieved an impressive 40% reduction in hospital readmissions due to prompt interventions. Concurrently, EDC systems revolutionize data collection by minimizing errors associated with manual entry, reducing human mistakes by as much as 30% through automated data input and programmed edit checks, ultimately leading to more accurate and reliable outcomes.
Organizations that adopt EDC technologies have reported average reductions of up to 30% in research timelines compared to traditional methods, underscoring the efficiency gains associated with these systems. Moreover, EDC systems foster collaboration among stakeholders by providing simultaneous access to a centralized database, which is essential for effective communication, decision-making, and monitoring in clinical trials.
To fully capitalize on these advancements, comprehensive training for staff on these technologies is imperative, ensuring seamless implementation and maximizing their benefits. As Jerry C Parker remarked, 'The adoption of EDC technologies is transforming the landscape of medical studies.
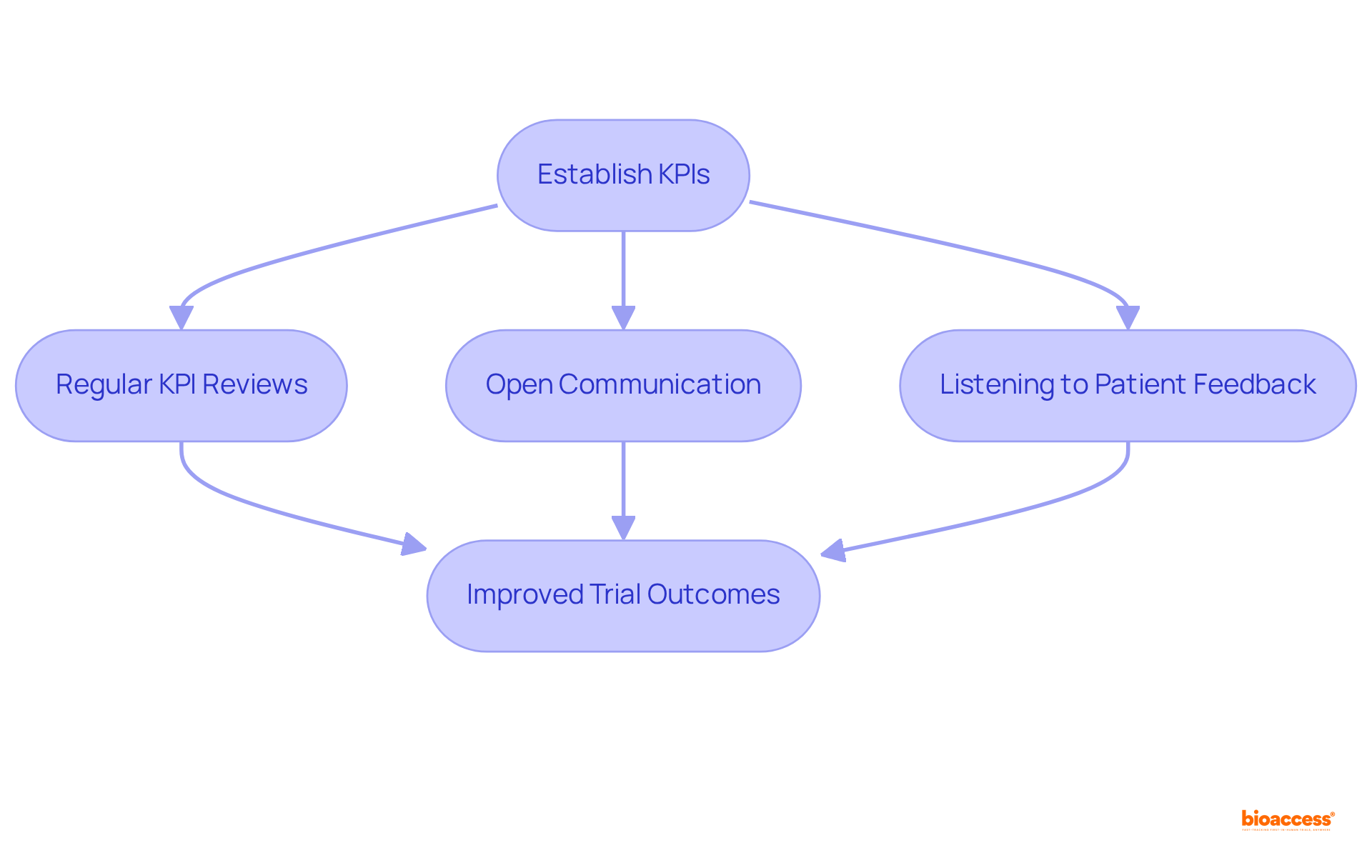
To implement effective monitoring in clinical trials, research managers must establish clear key performance indicators (KPIs) that align with the project's objectives. Regular reviews of these KPIs are crucial, as they enable the identification of trends and potential issues before they escalate. Open communication channels among team members and stakeholders facilitate timely reporting of concerns and collaborative problem-solving.
As Don Berwick remarked, "Listening to patients and integrating their feedback is essential to improving patient-centered care," which underscores the significance of communication in managing research studies. For instance, a clinical study for a new oncology medication that instituted weekly team meetings to discuss progress and challenges experienced a remarkable 25% increase in patient recruitment rates.
Moreover, bioaccess® provides ethical approvals in 4-6 weeks and secures enrollment 50% quicker than conventional markets, showcasing the effectiveness of efficient oversight strategies. Employing centralized monitoring in clinical trials improves oversight by offering a comprehensive view of study data, which enables proactive risk management and guarantees compliance with timelines.
This structured approach not only enhances the efficiency of clinical trials but also significantly contributes to better outcomes by incorporating effective monitoring in clinical trials.
Establishing effective monitoring practices in clinical trials is crucial for ensuring compliance, enhancing safety, and achieving optimal outcomes. By focusing on compliance standards, innovative monitoring techniques, and strategic oversight, organizations can significantly improve the integrity and reliability of their research studies. A commitment to these key practices not only fosters trust within the scientific community but also leads to more conclusive and reproducible results.
The article highlights three essential practices:
Each of these components plays a vital role in mitigating risks and promoting successful trial outcomes.
In a rapidly evolving regulatory landscape, the importance of proactive monitoring cannot be overstated. As organizations prepare for upcoming changes in compliance standards, embracing these best practices will not only facilitate adherence to regulations but also drive advancements in clinical research. The call to action is clear: prioritize effective monitoring to ensure the success of clinical trials, ultimately benefiting patients and the broader healthcare community.
What are the key regulatory frameworks for clinical trial monitoring?
The key regulatory frameworks for clinical trial monitoring include the Good Clinical Practice (GCP) guidelines and local regulations.
What is the first step in establishing compliance standards for clinical trial monitoring?
The first step is to create a comprehensive compliance checklist that includes essential elements such as informed consent processes, data integrity, and safety reporting.
Why are regular training sessions important for the research team?
Regular training sessions are vital to ensure that all team members understand their responsibilities and the importance of adhering to compliance standards, especially as the regulatory landscape evolves.
What role does auditing play in clinical trial monitoring?
Implementing a robust auditing process is essential for identifying compliance gaps early and enabling timely corrective actions, which helps maintain the integrity and reliability of the study.
How effective are regular compliance audits in clinical trials?
A study conducted by a notable biopharma firm showed that regular compliance audits resulted in a 30% decrease in protocol deviations, significantly enhancing the study's integrity and reliability.
What are the consequences of non-compliance in clinical trials?
Non-compliance can directly affect the validity and reliability of study results, potentially rendering them inconclusive.
Why is it important to stay informed about GCP guidelines?
Staying informed about GCP guidelines is essential because they continue to evolve, with updates scheduled to begin in July 2025. Proactive compliance efforts are necessary for the success of research studies.
How does adherence to compliance standards benefit the scientific community?
Adherence to compliance standards boosts the credibility of experiments and strengthens the scientific community's trust by enhancing the reproducibility of results.