


The article outlines best practices for ensuring compliance with clinical trial data security, emphasizing the critical importance of understanding regulatory frameworks. It highlights the necessity of implementing comprehensive security measures, training staff effectively, and leveraging advanced technology. These practices are supported by detailed recommendations, including:
Ensuring the security of clinical trial data is not merely a regulatory requirement; it is a fundamental aspect of maintaining trust and integrity in medical research. As organizations navigate the complex landscape of regulations such as HIPAA and GDPR, the stakes for protecting sensitive patient information have never been higher.
This article explores best practices for achieving compliance and safeguarding data, emphasizing critical measures that organizations can implement. With evolving technologies and increasing threats, the pressing question remains: how can clinical trials effectively balance innovation with security?
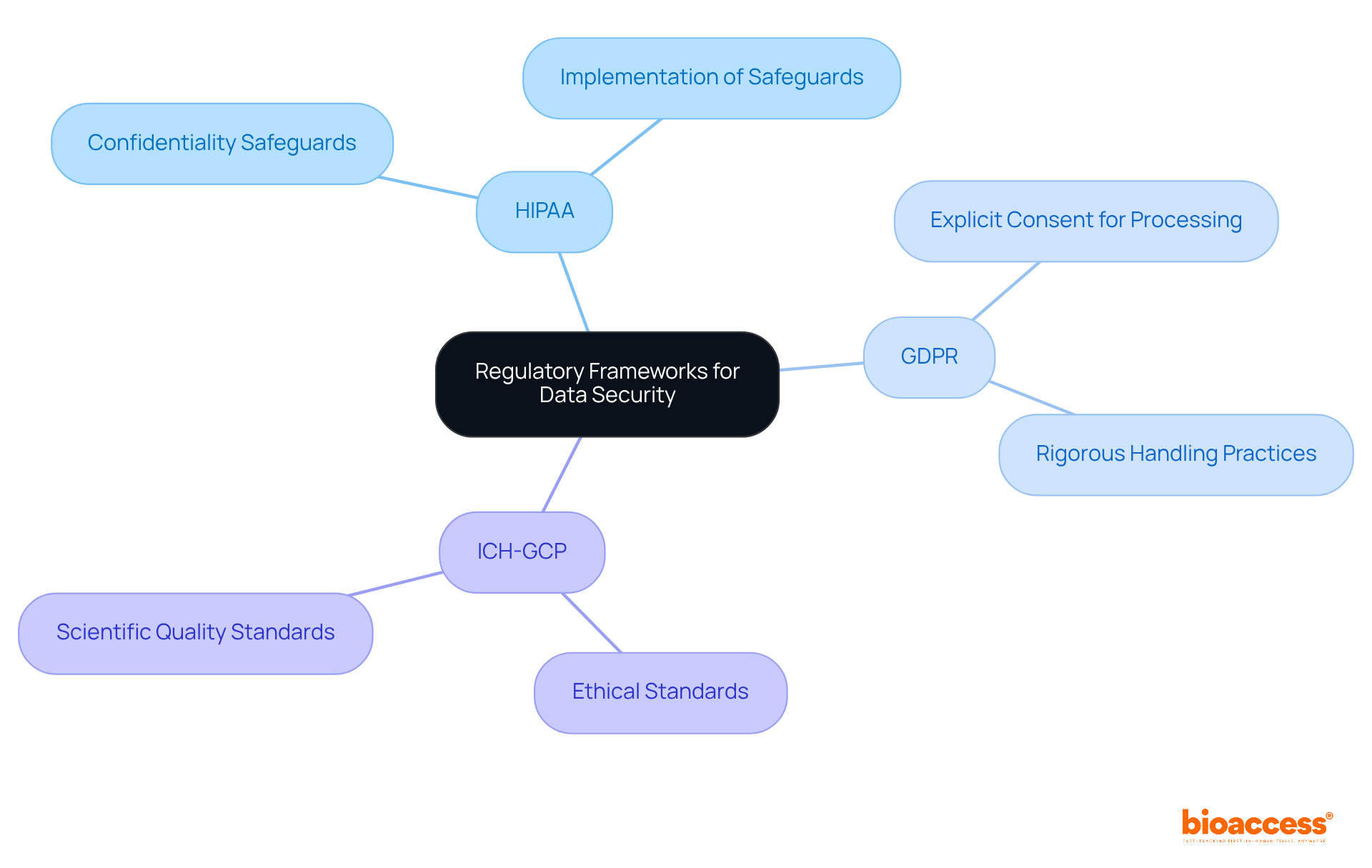
To effectively ensure clinical trial data security, understanding the regulatory frameworks that govern information protection is essential. Key regulations include:
Organizations like bioaccess offer extensive clinical study management services, including feasibility studies, site selection, compliance reviews, study setup, import permits, project management, and reporting. They also provide review and feedback on study documents to ensure compliance with country requirements and to monitor study progress. Regularly reviewing these regulations is crucial for maintaining compliance with clinical trial data security and avoiding potential legal repercussions. Engaging legal experts or compliance officers can further enhance understanding and adherence to these frameworks.
To safeguard sensitive clinical trial data, organizations must adopt a multi-layered security approach encompassing several critical measures:
By embracing these measures, organizations can markedly reduce the risk of information breaches while ensuring clinical trial data security and compliance with regulatory requirements.

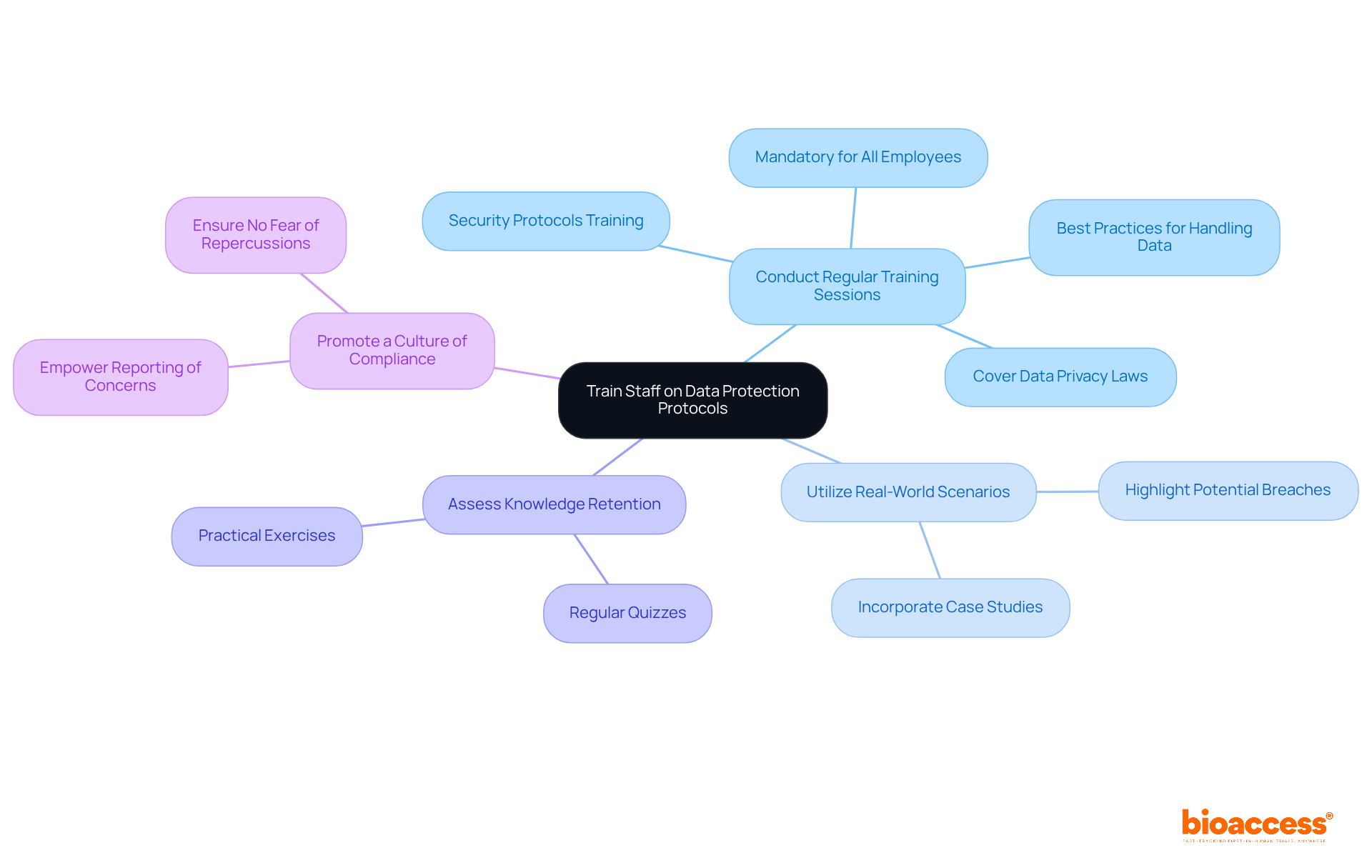
Educating personnel on information protection procedures is crucial for maintaining confidentiality and upholding clinical trial data security in clinical studies. Organizations must prioritize this initiative by implementing several key strategies:
By prioritizing employee training, companies can significantly enhance their overall information protection stance and ensure compliance with regulatory obligations.
Organizations can significantly enhance data security by leveraging advanced technologies, including:
By integrating these technologies, organizations can significantly improve their data security measures, particularly focusing on clinical trial data security, ensuring compliance and protecting sensitive information.

Ensuring the security of clinical trial data is paramount in today's regulatory landscape. Organizations must navigate a complex web of regulations such as HIPAA, GDPR, and ICH-GCP to protect sensitive information effectively. By understanding and adhering to these frameworks, companies can maintain compliance and safeguard participant confidentiality, ultimately enhancing trust in the clinical research process.
The article highlights several best practices that are essential for achieving robust clinical trial data security. These include:
The significance of prioritizing data security in clinical trials cannot be overstated. As the landscape of clinical research evolves, organizations must commit to continuous improvement in their security practices. By adopting these best practices and remaining vigilant in the face of emerging threats, stakeholders can ensure that clinical trial data remains secure, thereby protecting both participants and the integrity of the research itself.
What are the key regulatory frameworks for data security in clinical trials?
The key regulatory frameworks include HIPAA, GDPR, and ICH-GCP. HIPAA mandates the protection of patient health information in the U.S., GDPR focuses on information protection and privacy for organizations in the EU or dealing with EU citizens, and ICH-GCP outlines ethical and scientific quality standards for clinical studies.
What does HIPAA require from organizations regarding patient information?
HIPAA requires organizations to implement safeguards to ensure the confidentiality and protection of patient health information.
What is the purpose of the GDPR?
The GDPR emphasizes information protection and privacy, requiring organizations to obtain explicit consent for processing personal data and to handle information rigorously.
What does ICH-GCP stand for, and what does it ensure?
ICH-GCP stands for the International Council for Harmonisation - Good Clinical Practice. It ensures that ethical and scientific quality standards are upheld in the design, conduct, recording, and reporting of clinical studies, prioritizing information integrity and participant safety.
How can organizations ensure compliance with these regulatory frameworks?
Organizations can ensure compliance by regularly reviewing the regulations, engaging legal experts or compliance officers, and utilizing clinical study management services that offer feasibility studies, site selection, compliance reviews, and project management.
What services do organizations like bioaccess provide?
Organizations like bioaccess provide extensive clinical study management services, including feasibility studies, site selection, compliance reviews, study setup, import permits, project management, and reporting, as well as review and feedback on study documents to ensure compliance with country requirements.