


Quality control stands as the backbone of reliable healthcare delivery, especially within the realm of Point-of-Care Testing (PoCT) systems across Australia. By implementing best practices, organizations can significantly enhance patient safety and streamline operational efficiency. Yet, the myriad challenges of maintaining consistent quality across multiple sites raises a critical question: what are the most effective strategies for ensuring excellence in testing outcomes? This article explores essential practices that can strengthen quality control measures, ultimately leading to improved patient care and outcomes.
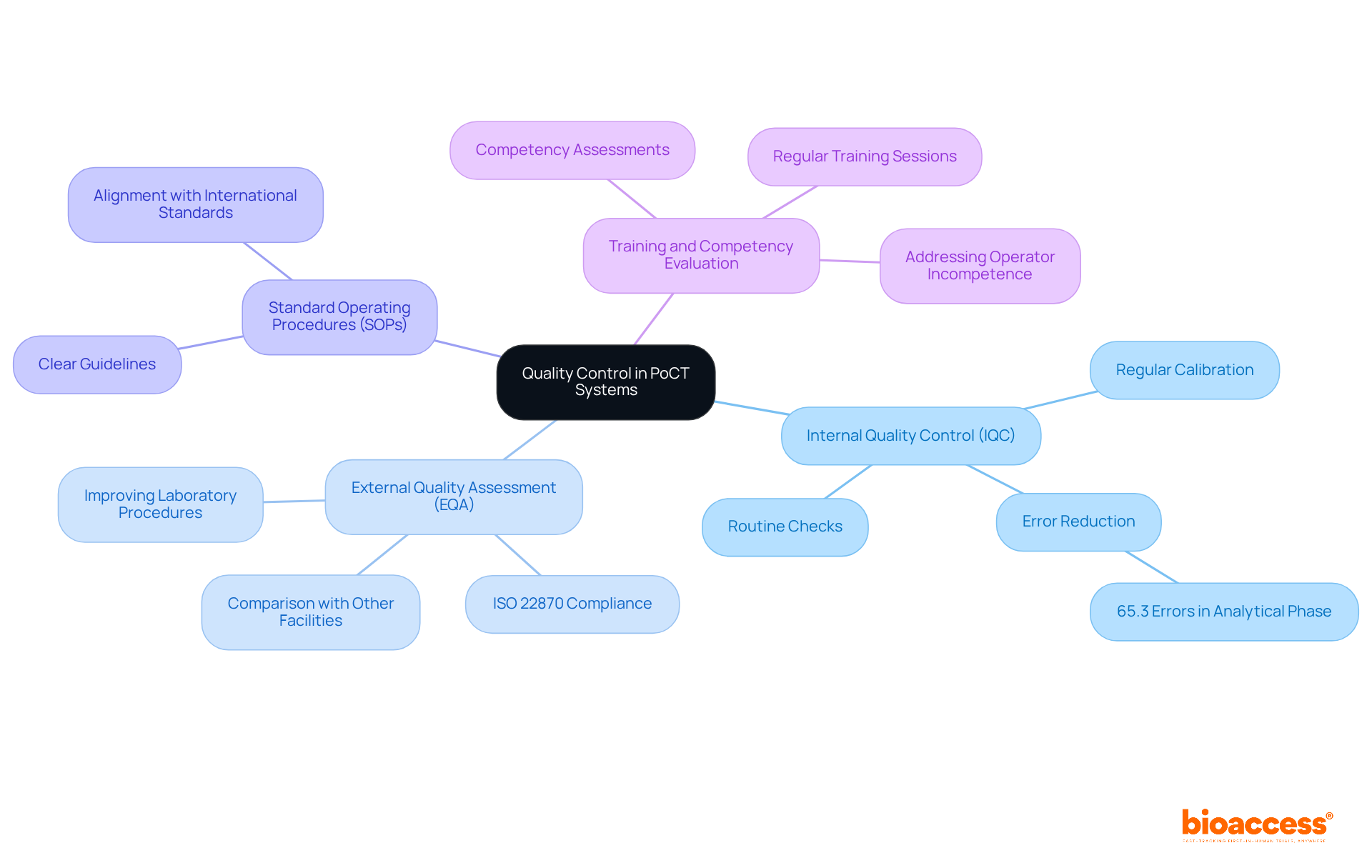
Quality control across sites in Australia is essential for ensuring precise and dependable outcomes in Point-of-Care Testing (PoCT) systems. Understanding the fundamentals of quality control can significantly enhance patient care and safety.
Internal Quality Control (IQC): Routine checks of testing processes and equipment are crucial to ensure proper functionality. Regular calibration and upkeep of devices greatly lower the chance of mistakes. In fact, studies show that 65.3% of errors in point-of-care testing occur during the analytical phase. By implementing stringent IQC measures, organizations can enhance patient safety and achieve quality control across sites in Australia, thereby mitigating these risks.
External Quality Assessment (EQA): Participation in EQA programs is not only beneficial but also mandated by ISO 22870 for all PoCT devices. This allows laboratories to compare their results with those from other facilities, promoting consistency and reliability across various evaluation sites. Effective EQA programs, such as those centered on Anaplastic Lymphoma Kinase (ALK) evaluation, have demonstrated their ability to uncover inconsistencies in examination methods, ultimately enhancing laboratory procedures and patient outcomes.
Standard Operating Procedures (SOPs): Establishing clear SOPs for all testing processes is essential for maintaining uniformity and excellence across different operators and locations. These procedures should align with international standards, ensuring quality control across sites in Australia and adherence to quality management methods.
Training and Competency Evaluation: Regular training sessions for personnel involved in point-of-care testing are vital to keep them informed about the latest practices and technologies. Continuous education is essential for upholding high-quality standards, as operator incompetence is a major source of error in point-of-care testing. Implementing competency assessments can further ensure that personnel are equipped to perform tests accurately and reliably.
By comprehending and applying these basics, organizations can create a strong foundation for their control efforts in point-of-care testing. The potential impact of control errors on patient outcomes is considerable, necessitating rigorous control measures. Are you ready to enhance the reliability of your test outcomes and improve patient care?
To establish a robust quality framework for Point-of-Care Testing (PoCT), organizations must implement essential best practices that not only enhance operational efficiency but also ensure patient safety and satisfaction.
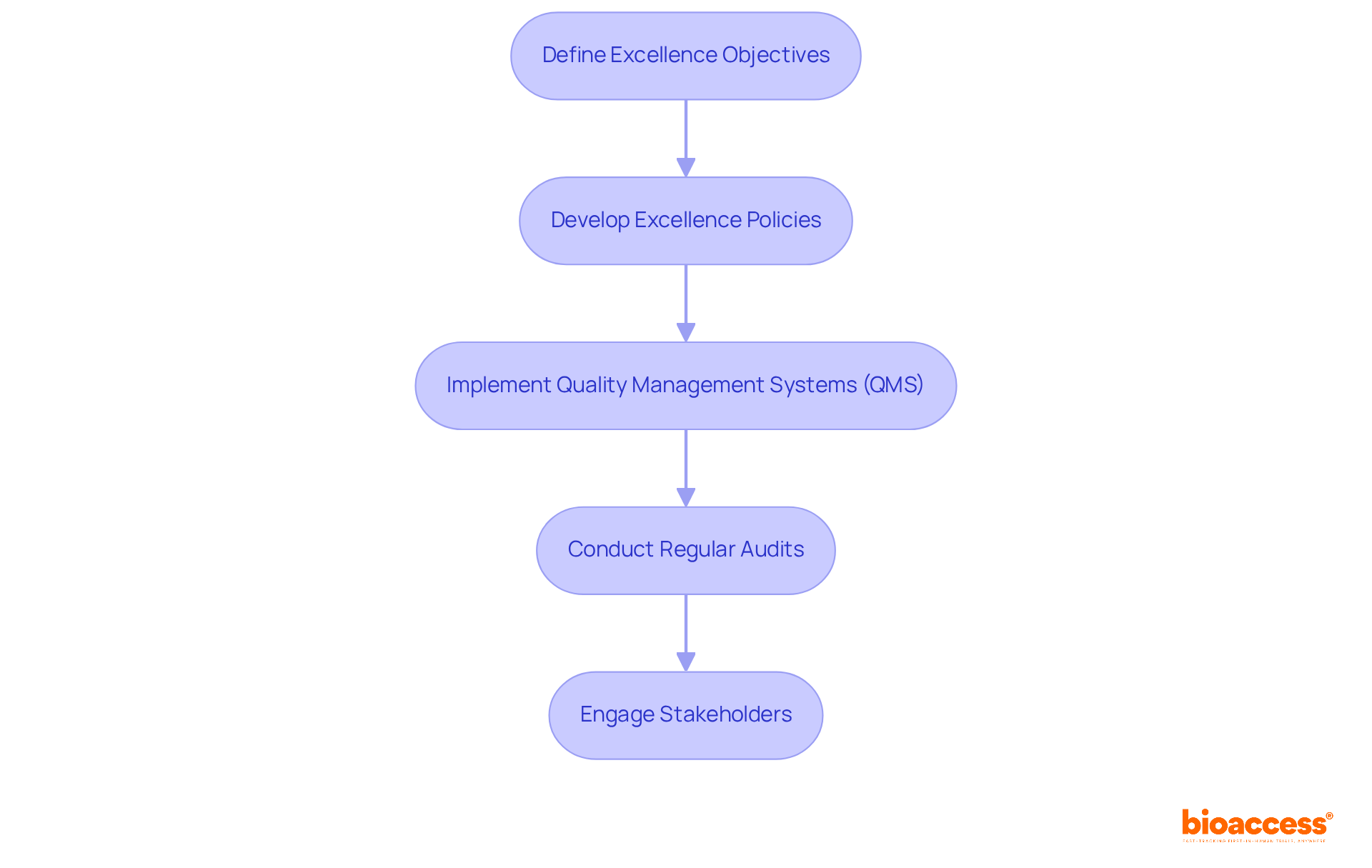
Define Excellence Objectives: Start by clearly articulating excellence objectives that align with your organizational goals and regulatory standards. Focus on accuracy, reliability, and patient safety. According to ISO 9001:2015, these objectives should be suitable to the policy, measurable, and centered on product conformity and customer satisfaction. Setting these objectives is crucial for directing all management efforts regarding standards.
Develop Excellence Policies: Next, formulate comprehensive policies that govern standards management practices. These policies should include detailed procedures for testing, reporting, and addressing issues, ensuring consistency and compliance across all operations.
Implement Quality Management Systems (QMS): Adopt a QMS that integrates all quality-related processes. This system should guarantee that all procedures are recorded, observed, and open to ongoing enhancement, fostering a culture of excellence throughout the organization.
Conduct Regular Audits: Schedule internal audits to assess adherence to established standards and identify areas for improvement. This proactive approach not only helps uphold high standards but also reinforces a commitment to excellence at every level of the organization.
Engage Stakeholders: Actively involve all relevant stakeholders, including laboratory personnel, management, and regulatory bodies, in the development and implementation of the standards framework. This engagement is vital for ensuring buy-in and adherence to excellence standards. Additionally, having a dedicated team of specialists, such as POCT Coordinators, can significantly enhance the effectiveness of these practices by overseeing daily operations and facilitating communication among stakeholders.
By adhering to these steps, organizations can establish a thorough standards framework that boosts the effectiveness of point-of-care testing operations, ultimately leading to improved patient outcomes and operational efficiency.
To implement effective external quality assurance checks in Point-of-Care Testing (PoCT), organizations must adopt the following best practices:
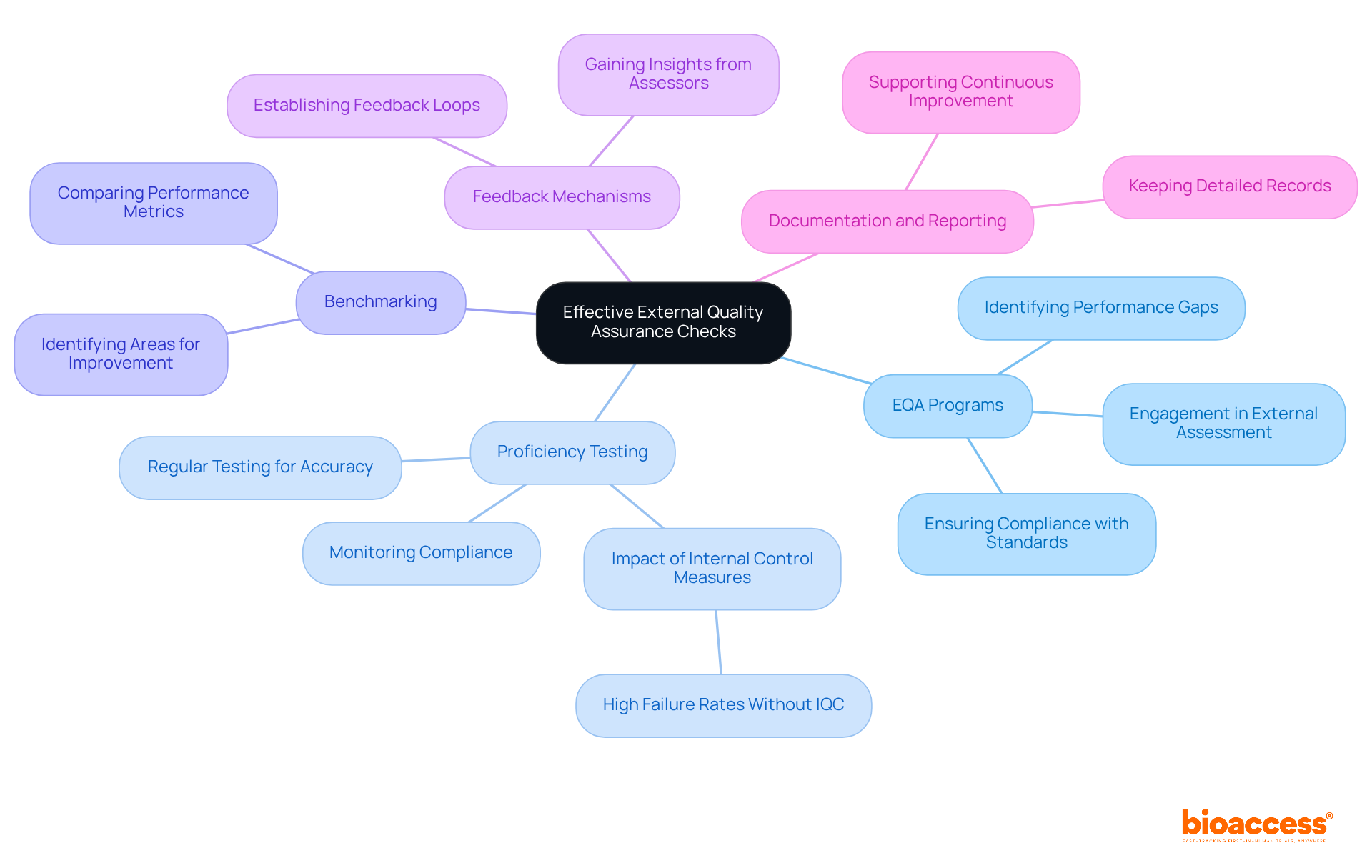
Participate in EQA Programs: Engaging in external assessment (EQA) programs is essential for obtaining comparative data on test results across different laboratories. This participation helps identify performance gaps and areas for improvement, ensuring compliance with international standards.
Regular Proficiency Testing: Conducting proficiency testing (PT) is crucial for assessing the accuracy of testing processes. By periodically sending samples to participating laboratories, organizations can monitor ongoing compliance with performance standards and enhance their operational efficiency. Research indicates that laboratories lacking daily internal control measures are 3.8 times more likely to encounter high failure rates, underscoring the significance of regular PT.
Benchmarking: Organizations should compare their performance metrics against industry standards and best practices. This benchmarking process enables the recognition of specific areas requiring improvement, promoting a culture of continuous excellence enhancement.
Feedback Mechanisms: Establishing feedback loops with external assessors is vital for gaining insights into performance and identifying areas requiring attention. This proactive strategy can improve testing procedures and enhance overall standards, ultimately leading to better patient outcomes.
Documentation and Reporting: Keeping detailed records of all external assurance activities, including outcomes and corrective measures taken, is crucial. This practice not only aids in compliance but also supports continuous quality improvement efforts, ensuring that organizations can effectively manage risks and uphold their reputation. As highlighted by LGC AXIO Proficiency Evaluation, proficiency assessment is a crucial instrument for ensuring that your laboratory obtains its results accurately-both currently and in the future.
By applying these strategies, organizations can significantly improve the dependability and precision of their point-of-care test results, ultimately aiding in better healthcare delivery.
Establishing clear roles for testing and evaluation in Point-of-Care Testing (PoCT) is crucial for effective management of standards. Organizations must prioritize this to enhance accountability and streamline quality management processes.
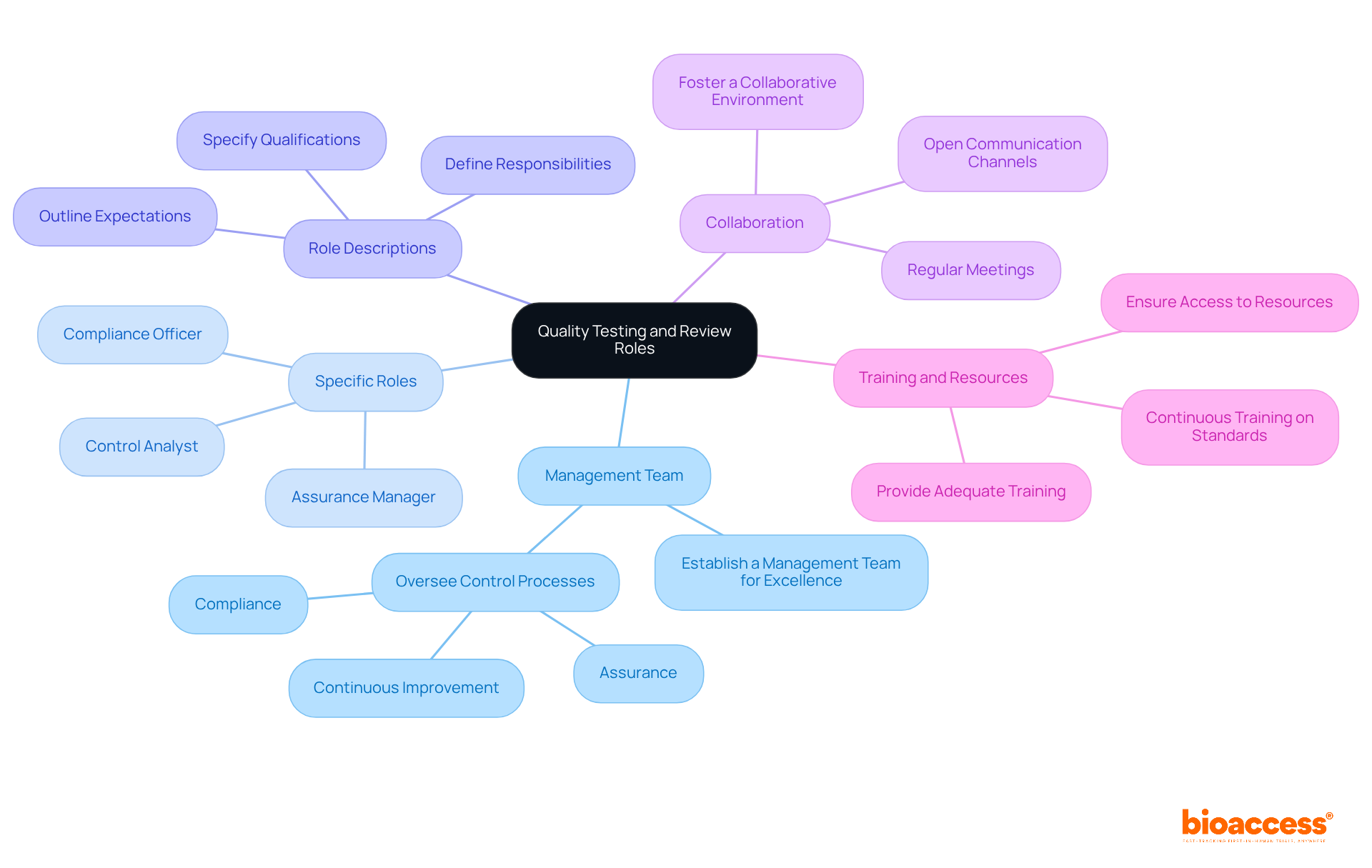
Establish a Management Team for Excellence: Form a dedicated team responsible for overseeing control processes, including assurance, compliance, and continuous improvement initiatives. This team is vital for maintaining high standards in PoCT.
Assign Specific Roles: Clearly delineate roles within the assurance management team, such as Assurance Manager, Control Analyst, and Compliance Officer. Each member should fully understand their responsibilities to ensure smooth operations.
Develop Role Descriptions: Create detailed role descriptions that outline expectations, responsibilities, and required qualifications for each position. This clarity aids in recruitment and training efforts, ensuring that the right individuals are in place.
Encourage Collaboration: Foster a collaborative environment where team members can share insights and feedback on performance issues. Regular meetings and open communication channels enhance teamwork and drive continuous improvement.
Provide Training and Resources: Ensure that all team members receive adequate training and resources to perform their roles effectively. Continuous training on standards and best practices is essential for maintaining excellence in PoCT.
Ensuring effective quality control across sites in Australia is crucial for achieving reliable outcomes in Point-of-Care Testing (PoCT) systems. Implementing best practices - such as internal quality control, external quality assessments, standardized operating procedures, and robust training programs - can significantly enhance patient care and safety. A well-structured approach to quality management minimizes errors and fosters a culture of continuous improvement.
Key insights from this discussion highlight the necessity of:
Regular audits and stakeholder engagement reinforce the commitment to high standards and operational efficiency. By embracing these practices, healthcare organizations can ensure they meet both regulatory requirements and patient expectations.
The importance of rigorous quality control measures cannot be overstated. As healthcare continues to evolve, organizations must remain vigilant and proactive in their quality assurance efforts. This vigilance not only improves testing accuracy and reliability but also enhances overall patient outcomes, paving the way for a safer and more effective healthcare environment. Embracing these best practices today prepares organizations for the challenges of tomorrow, ensuring that quality remains at the forefront of patient care.
Why is quality control important in Point-of-Care Testing (PoCT) systems?
Quality control is essential in PoCT systems to ensure precise and dependable outcomes, which significantly enhances patient care and safety.
What is Internal Quality Control (IQC) and why is it necessary?
Internal Quality Control (IQC) involves routine checks of testing processes and equipment to ensure proper functionality. It is necessary to reduce the chance of mistakes, as studies show that a significant percentage of errors in PoCT occur during the analytical phase.
What percentage of errors in point-of-care testing occur during the analytical phase?
Studies indicate that 65.3% of errors in point-of-care testing occur during the analytical phase.
What is External Quality Assessment (EQA) and why is it important?
External Quality Assessment (EQA) involves participating in programs that allow laboratories to compare their results with those from other facilities. It is important for promoting consistency and reliability across various evaluation sites and is mandated by ISO 22870 for all PoCT devices.
How do effective EQA programs improve laboratory procedures?
Effective EQA programs can uncover inconsistencies in examination methods, which ultimately enhances laboratory procedures and improves patient outcomes.
What are Standard Operating Procedures (SOPs) and their role in quality control?
Standard Operating Procedures (SOPs) are clear guidelines established for all testing processes. They are crucial for maintaining uniformity and excellence across different operators and locations, ensuring adherence to international standards.
Why is training and competency evaluation important in PoCT?
Training and competency evaluation are vital to keep personnel informed about the latest practices and technologies. Regular training helps uphold high-quality standards, as operator incompetence is a major source of error in point-of-care testing.
What can organizations do to enhance their quality control efforts in PoCT?
Organizations can enhance their quality control efforts by understanding and applying the fundamentals of IQC, EQA, SOPs, and regular training for personnel, thereby creating a strong foundation for reliable test outcomes and improved patient care.