


The article outlines four essential steps for the Premarket Approval (PMA) process of medical devices, underscoring the critical importance of thorough clinical data and regulatory compliance to ensure product safety and efficacy. It captures attention by detailing each step—
while also addressing significant challenges such as lengthy approval times and high costs. Furthermore, it suggests proactive strategies to navigate these obstacles effectively, reinforcing the necessity of a well-structured approach in the Medtech landscape.
Navigating the complex landscape of medical device regulation can be daunting, particularly when it comes to Premarket Approval (PMA), a critical process mandated by the FDA for high-risk medical instruments. This rigorous evaluation not only safeguards public health but also propels innovation within the medical technology sector. However, the intricacies of the PMA process can pose significant challenges for manufacturers striving to bring their products to market. What essential steps can streamline this journey and enhance the likelihood of successful approval?
Premarket Approval (PMA for medical devices) represents a rigorous regulatory process established by the U.S. Food and Drug Administration (FDA) specifically for high-risk medical instruments. Unlike the 510(k) pathway, which allows products to enter the market based on significant similarity to existing offerings, the PMA for medical devices requires that producers supply comprehensive clinical information demonstrating the safety and efficacy of their items. This thorough assessment is essential for safeguarding public health, ensuring that only products meeting high safety and efficacy standards are authorized for use.
The significance of PMA for medical devices transcends mere regulatory compliance; it actively fosters innovation within the medical technology sector while simultaneously cultivating trust among healthcare providers and patients. By guaranteeing that products undergo exhaustive evaluations prior to market introduction, the PMA for medical devices enhances the overall quality of medical equipment available to the public.
Recent updates to the PMA for medical devices reflect ongoing efforts to improve regulatory efficiency. Notably, the FDA has set a target for 90% of original PMAs to receive a decision within 180 days, as outlined under the Medical Device User Fee Amendments (MDUFA IV). This streamlined approach not only simplifies the approval process but also reinforces the FDA's commitment to ensuring equipment safety.
Real-world examples illustrate the protective role of PMA in public health. The FDA's comprehensive evaluations have historically resulted in a high approval rate for PMAs, with nearly all applications accepted in recent years. This meticulous examination is particularly crucial in scenarios where the safety of the equipment is paramount, as evidenced by the FDA's proactive measures to address adverse occurrences related to medical tools.
In this context, firms like bioaccess® play a pivotal role in enhancing medical equipment through the PMA for medical devices process. With over 20 years of expertise in Medtech clinical trials, bioaccess® specializes in Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF). Their tailored approach not only facilitates navigation through the PMA for medical devices process but also ensures that startups in the Medtech, Biopharma, and Radiopharma sectors can effectively meet regulatory standards, ultimately accelerating their pathway to approval.
In summary, the PMA for medical devices procedure is vital for upholding high standards in medical equipment safety, fostering innovation, and ensuring that healthcare professionals and patients can trust the tools they rely on for their health and well-being.
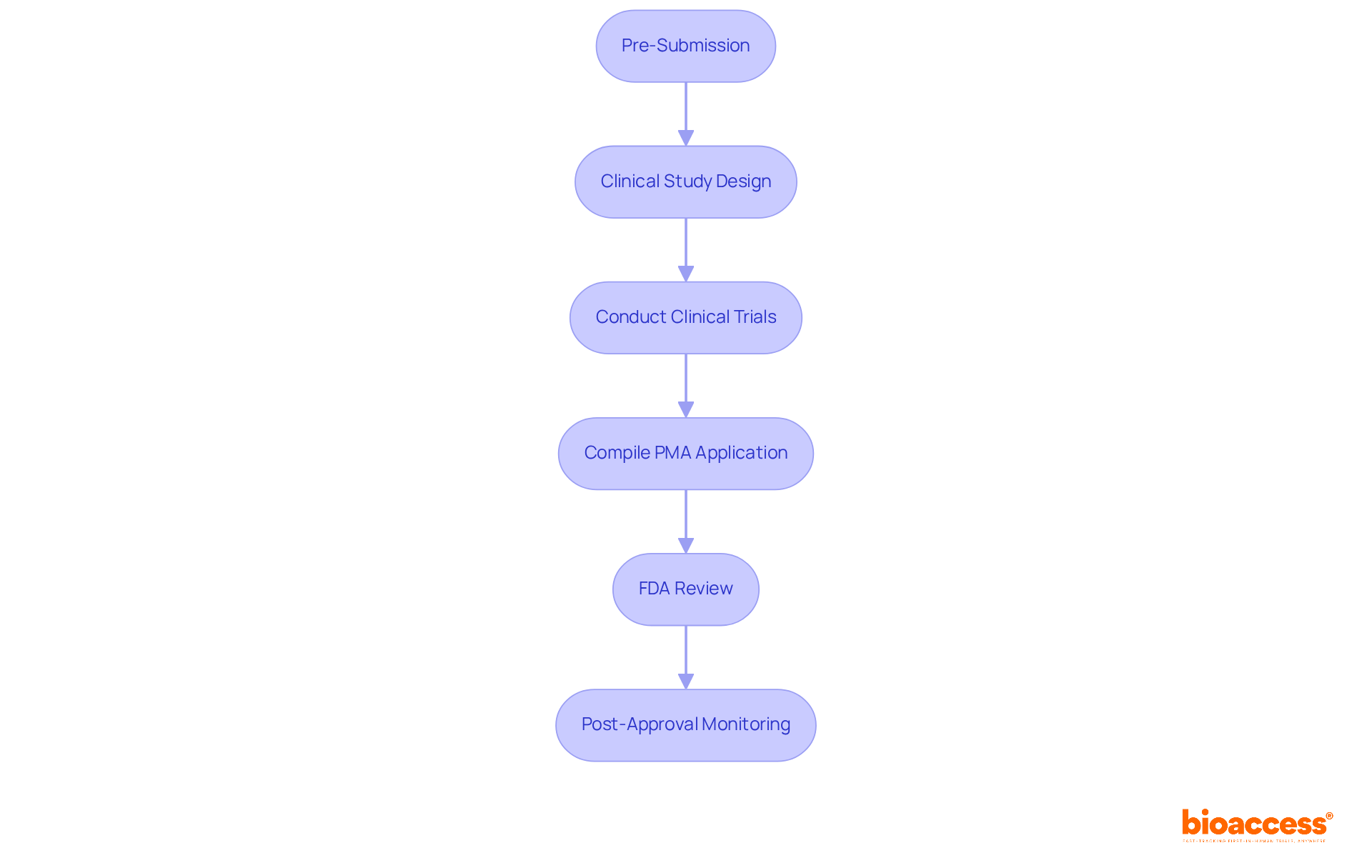
The procedure known as PMA for medical devices is essential for producers aiming to introduce high-risk medical instruments to the market. This process encompasses several key steps:
Pre-Submission: Early engagement with the FDA is critical. This initial step allows producers to discuss their proposed apparatus and receive input on the necessary data requirements, significantly simplifying the submission process.
Clinical Study Design: A robust clinical study plan is vital. This plan should clearly outline the study's objectives, methodology, and endpoints to effectively demonstrate the safety and effectiveness of the apparatus. Successful PMA submissions often feature well-structured clinical trials that meet FDA expectations.
Conduct Clinical Trials: Clinical trials must be executed in accordance with the approved protocol, ensuring compliance with Good Clinical Practice (GCP) guidelines. Adhering to these guidelines is crucial for maintaining the integrity of the data collected.
Compile PMA Application: It is important to gather all necessary documentation, including clinical data, manufacturing information, and labeling, to create a comprehensive PMA application. A well-structured application facilitates a more seamless review process.
FDA Review: After submission, the PMA application undergoes a thorough review by the FDA, which may include advisory committee meetings. The average review time for PMA applications is approximately 363.2 days, reflecting improvements from previous years.
Post-Approval Monitoring: Following approval, manufacturers must continue to monitor the product's performance and report any adverse events to the FDA. This ongoing vigilance is essential for maintaining compliance and ensuring patient safety.
Understanding these steps is crucial for manufacturers aiming for a successful PMA for medical devices submission. As noted by FDA officials, a strategic approach that integrates regulatory requirements from the outset can significantly enhance the likelihood of approval.
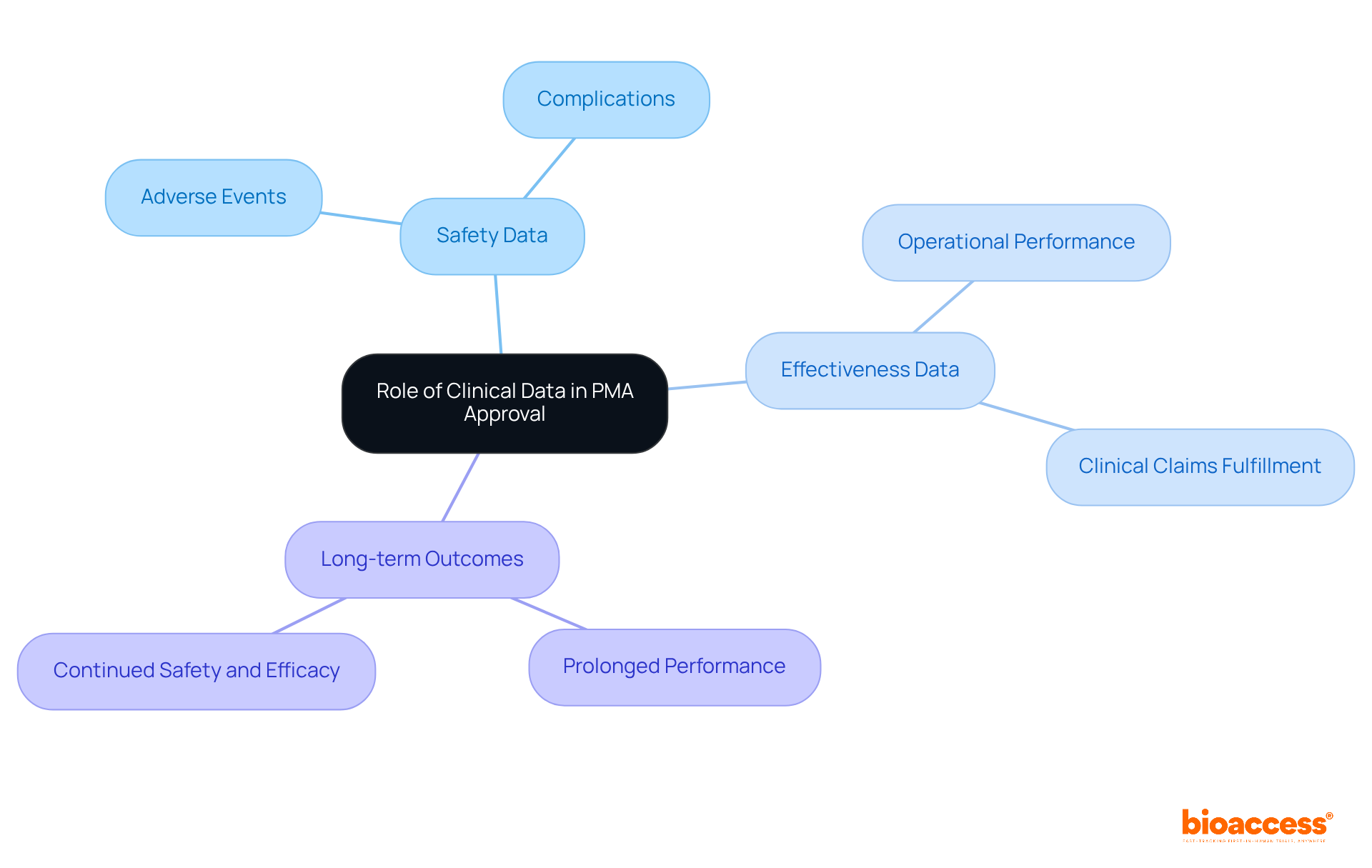
Clinical data is paramount in the PMA for medical devices approval process, as it provides the essential evidence to demonstrate that a medical instrument is safe and effective for its intended use. This data is typically gathered through rigorously designed clinical trials that comply with regulatory standards. With bioaccess®, manufacturers can achieve 50% faster patient enrollment and realize $25K savings per patient through FDA-ready data, effectively eliminating rework and delays. The types of clinical data required include:
Manufacturers must ensure that clinical data is collected, analyzed, and presented in a manner that meets FDA expectations, as this significantly influences the likelihood of obtaining PMA for medical devices approval. For instance, Avantec Vascular's initial human clinical trial of a novel vascular device in Latin America illustrates how bioaccess® aids in selecting lead investigators and regulatory applications, simplifying the approval pathway for startups.
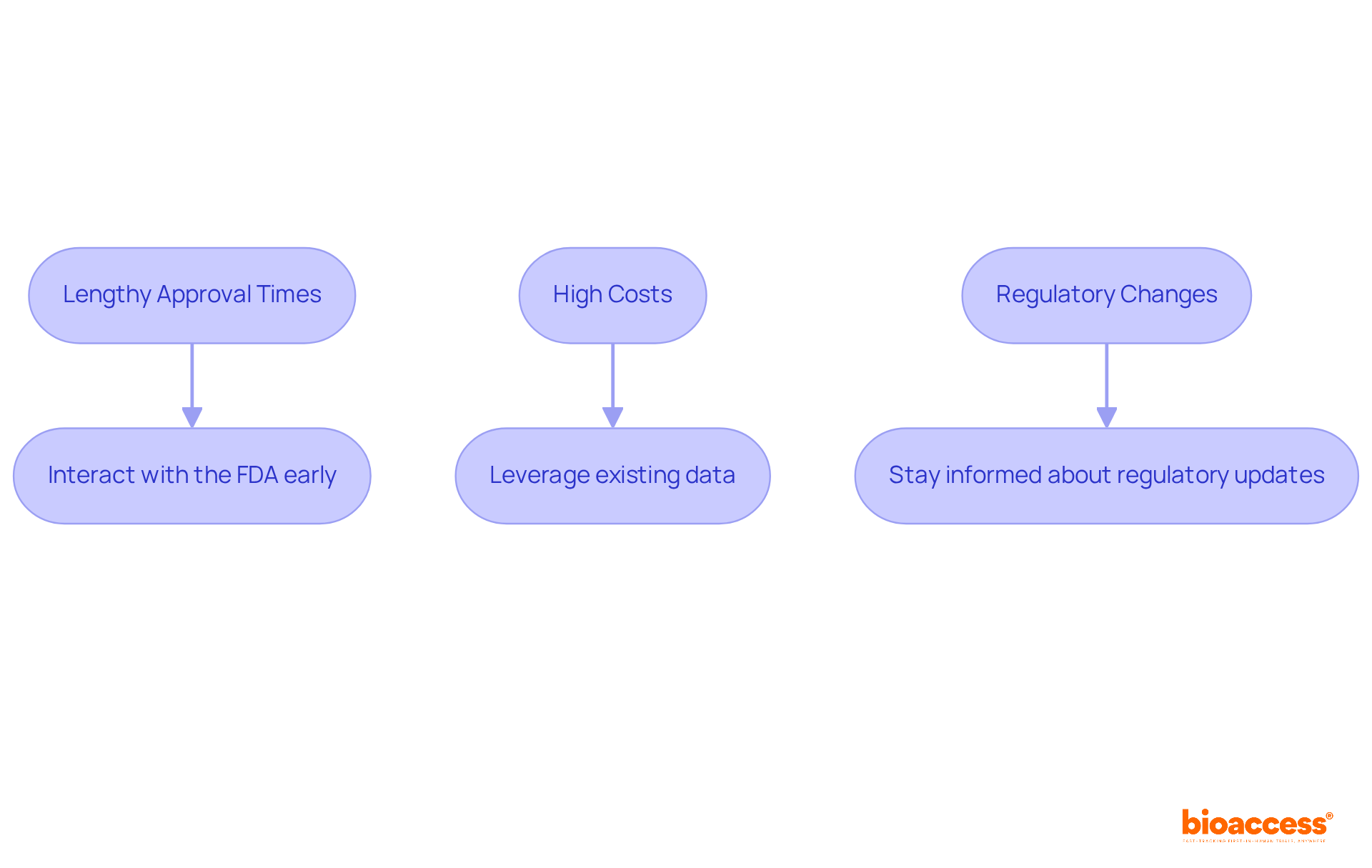
The challenges presented by the PMA for medical devices process require manufacturers to navigate them effectively to ensure successful product launches.
Lengthy Approval Times: The PMA process can take several months to years, potentially delaying product launch. Solution: Interact with the FDA early in the procedure to clarify requirements and streamline submissions.
High Costs: Conducting clinical trials and compiling PMA applications can be expensive. Solution: Consider leveraging existing data from previous studies or collaborating with other organizations to share costs.
Regulatory Changes: Evolving regulations can impact the PMA process. Solution: Stay informed about regulatory updates and adapt strategies accordingly.
By proactively addressing these challenges, manufacturers can enhance their chances of a successful submission for PMA for medical devices and expedite their time to market.
The Premarket Approval (PMA) process for medical devices stands as a critical regulatory framework, meticulously designed to ensure that high-risk medical instruments meet stringent safety and efficacy standards. By mandating comprehensive clinical data and thorough evaluations, the PMA not only safeguards public health but also nurtures innovation and cultivates trust among healthcare professionals and patients alike.
This article outlined key steps in the PMA process, which include:
Furthermore, the significance of clinical data in demonstrating a device's safety and effectiveness was underscored, alongside the challenges manufacturers encounter in navigating the PMA landscape. Solutions such as:
emerged as essential for overcoming these hurdles.
In conclusion, a comprehensive understanding of the PMA process is vital for manufacturers aspiring to introduce innovative medical devices to the market. By prioritizing regulatory compliance and focusing on high-quality clinical data, stakeholders can significantly enhance their chances of successful approval while contributing to the advancement of healthcare technology. Embracing these practices will ultimately lead to the development of safer, more effective medical devices that improve patient outcomes and foster a healthier society.
What is Premarket Approval (PMA) for medical devices?
Premarket Approval (PMA) is a rigorous regulatory process established by the U.S. Food and Drug Administration (FDA) for high-risk medical instruments. It requires manufacturers to provide comprehensive clinical information demonstrating the safety and efficacy of their products before they can enter the market.
How does PMA differ from the 510(k) pathway?
Unlike the 510(k) pathway, which allows products to enter the market based on significant similarity to existing devices, the PMA process requires a thorough assessment of clinical data to ensure that only products meeting high safety and efficacy standards are authorized for use.
Why is the PMA process important?
The PMA process is crucial for safeguarding public health by ensuring that medical devices undergo exhaustive evaluations before market introduction. It enhances the overall quality of medical equipment and fosters trust among healthcare providers and patients.
What recent updates have been made to the PMA process?
Recent updates include the FDA's target for 90% of original PMAs to receive a decision within 180 days, as part of the Medical Device User Fee Amendments (MDUFA IV). This aims to improve regulatory efficiency and streamline the approval process.
How does PMA protect public health?
The PMA process involves comprehensive evaluations that have historically resulted in a high approval rate for applications. This meticulous examination is essential in ensuring the safety of medical equipment and addressing any adverse occurrences related to medical tools.
What role do companies like bioaccess® play in the PMA process?
Companies like bioaccess® specialize in enhancing medical equipment through the PMA process. They provide expertise in various clinical trials and help startups in the Medtech, Biopharma, and Radiopharma sectors navigate regulatory standards, ultimately accelerating their pathway to approval.
What are the benefits of the PMA process for innovation?
The PMA process not only ensures high safety and efficacy standards but also actively fosters innovation within the medical technology sector, allowing for the development of advanced medical devices that can improve patient care.