


Effective site feasibility planning stands as a cornerstone of successful biopharma trials, especially in a region as dynamic as Serbia. Researchers navigating the complexities of local regulations, cultural nuances, and logistical challenges can gain invaluable insights that significantly enhance study outcomes. But how can one ensure that the chosen sites not only meet regulatory standards but also align with specific research objectives in this rapidly evolving landscape? By exploring key practices in site feasibility planning, we uncover strategies that can transform potential hurdles into stepping stones for success.
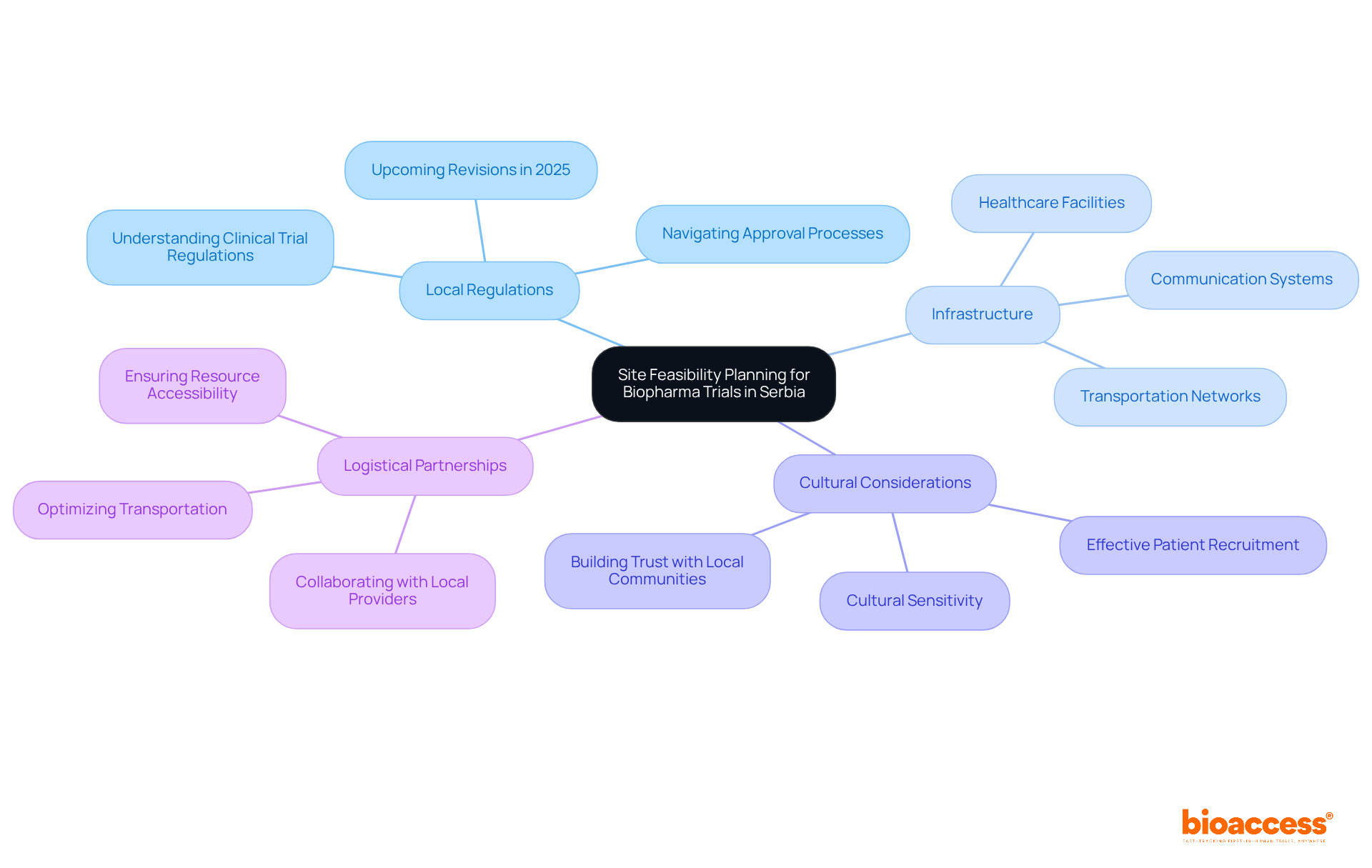
Effective site feasibility planning for biopharma trials in Serbia is crucial for successful clinical research, requiring a comprehensive understanding of the local context, including cultural, regulatory, and logistical factors.
Researching Local Regulations: Familiarizing yourself with Serbia's clinical trial regulations is essential, as they can differ significantly from those in other regions. A thorough grasp of these regulations not only aids in navigating the approval processes but also prepares you for the upcoming revisions in Serbia's research protocols in 2025, which aim to create a more supportive environment for investigation.
Assessing Infrastructure: Evaluating the availability and quality of local infrastructure-such as transportation networks, healthcare facilities, and communication systems-is vital. This assessment guides your choice of location and logistical preparations, ensuring that experiments can be conducted smoothly and efficiently.
Cultural Considerations: Understanding cultural differences is key for effective patient recruitment and retention. Engaging with local communities and appreciating their values can significantly enhance trust and participation in clinical studies. Experts agree that cultural sensitivity is essential for building positive relationships with potential participants.
Logistical Partnerships: Collaborating with local logistics providers is critical for optimizing transportation and access to research sites. Ensuring that all necessary resources and staff can reach these locations promptly is vital for maintaining timelines and operational effectiveness.
By integrating these components into your feasibility planning process, you can significantly boost the likelihood of successful execution in this region.
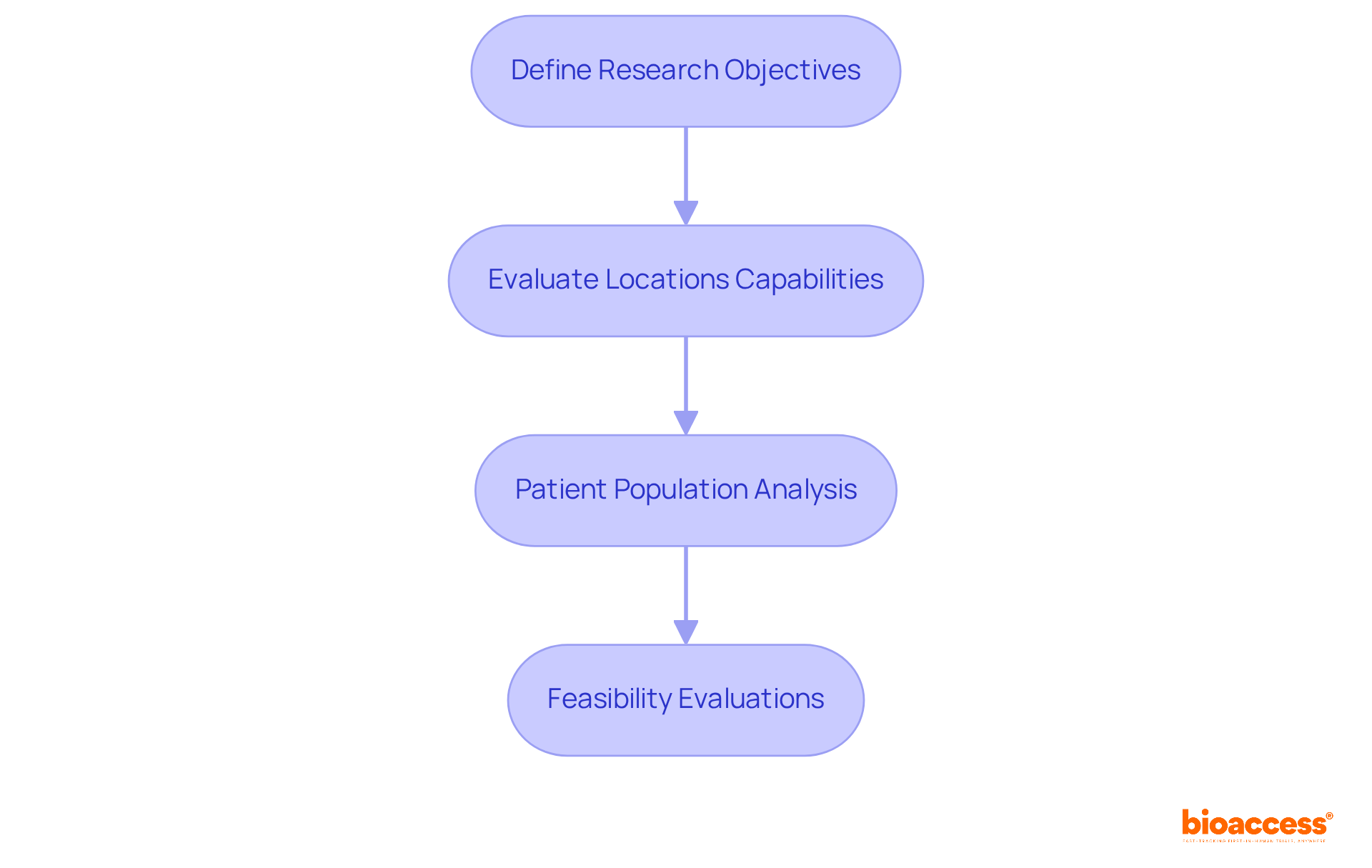
Choosing locations for clinical studies in Serbia necessitates site feasibility planning for biopharma trials to strategically align facility capabilities with specific research goals. This process is crucial for ensuring that studies are conducted efficiently and effectively.
Define Research Objectives: Start by clearly articulating the study's goals, including target populations and endpoints. This clarity is essential as it guides the selection of locations that can effectively meet these objectives.
Evaluate Locations Capabilities: Next, assess potential locations based on their resources, such as staff expertise, patient access, and prior experience with similar trials. Sites with a proven track record in relevant studies tend to be more reliable and efficient, enhancing the overall study quality.
Patient Population Analysis: Conduct a thorough examination of the demographics and prevalent health conditions in the area surrounding each location. Confirming that chosen sites can reach the necessary patient groups is vital, as successful recruitment hinges on this factor.
Feasibility Evaluations: Finally, carry out comprehensive feasibility evaluations that include location visits, discussions with personnel, and assessments of past performance metrics. This data-driven approach is crucial for site feasibility planning for biopharma trials in Serbia, as it helps identify the most appropriate locations for the study, ultimately improving the chances of effective patient recruitment and data gathering.
By adhering to these guidelines and leveraging bioaccess's extensive management services-including feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting-researchers can significantly enhance their likelihood of achieving study objectives in the region.
Effective communication with local stakeholders is crucial for site feasibility planning for biopharma trials in Serbia. By implementing targeted strategies, researchers can significantly enhance this communication, leading to more effective collaborations.
Identify Key Stakeholders: Recognizing all relevant parties - local healthcare providers, regulatory bodies, and community leaders - is essential. Understanding their roles and interests not only facilitates collaboration but also aligns goals effectively.
Establish Open Lines of Communication: Creating channels for regular updates and feedback, such as meetings, newsletters, or digital platforms, is vital. Keeping stakeholders informed about the project's progress fosters transparency and builds trust, which are key components of successful partnerships.
Engage in Community Outreach: Implementing outreach programs to educate the local community about the initiative's purpose and benefits is a proactive approach. This engagement not only enhances recruitment efforts but also establishes a foundation of trust, crucial for successful participation.
Address Concerns Promptly: Proactively addressing any concerns or questions raised by stakeholders is essential. Timely responses can prevent misunderstandings and strengthen relationships, ensuring a collaborative environment.
By prioritizing these communication strategies, researchers can cultivate a supportive atmosphere that significantly enhances site feasibility planning for biopharma trials in Serbia and improves overall study success.

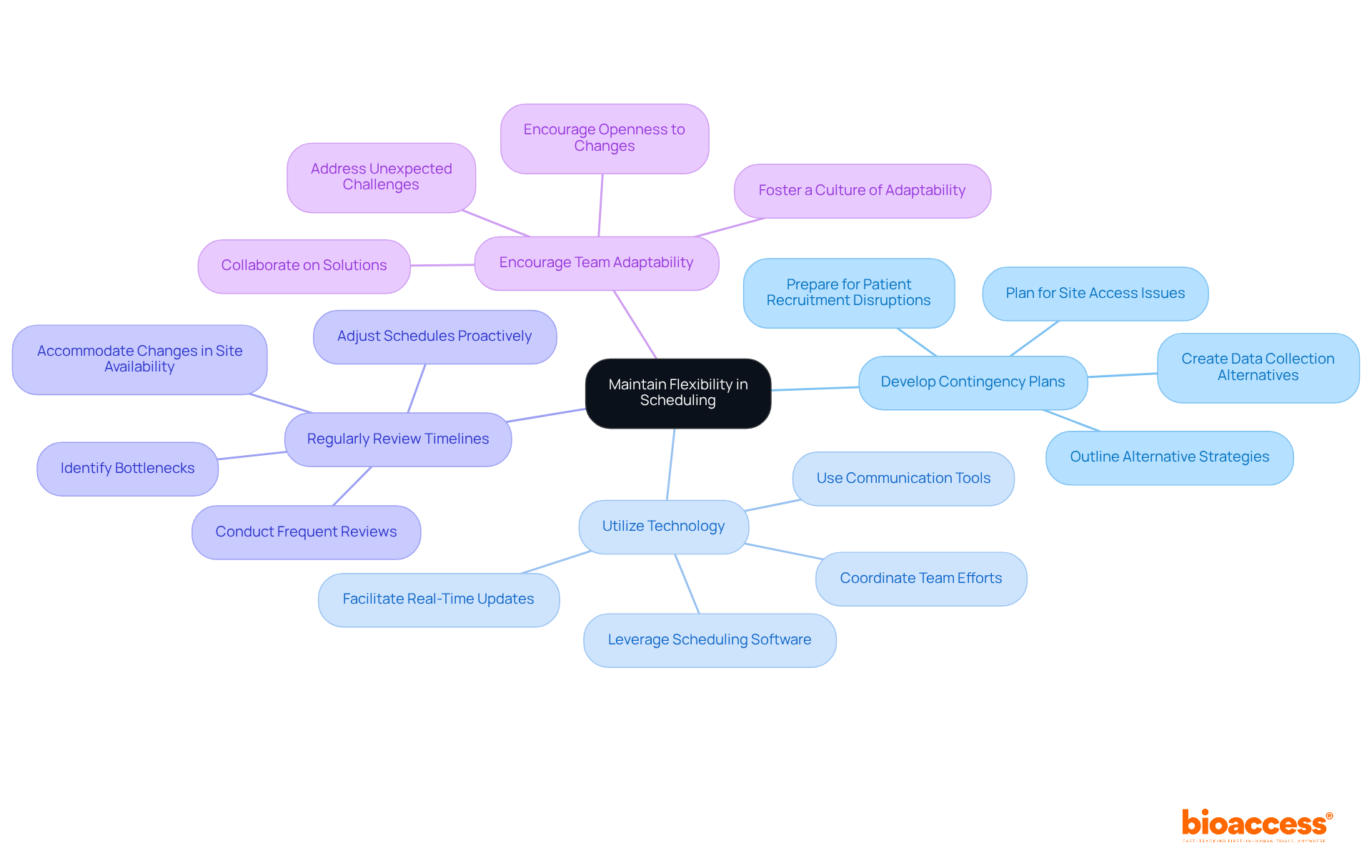
Flexibility in scheduling is crucial for effectively managing the complexities involved in site feasibility planning for biopharma trials in Serbia. To maintain this flexibility, consider the following strategies:
By implementing these strategies, researchers can significantly enhance their ability to navigate the complexities of clinical trial scheduling. This proactive approach ultimately leads to more successful outcomes.
Effective site feasibility planning in Serbia demands a multifaceted approach that integrates a profound understanding of local contexts, strategic site selection, robust stakeholder communication, and flexibility in scheduling. By prioritizing these elements, researchers can significantly enhance the likelihood of successful clinical trials in the biopharma sector, ultimately driving advancements in medical research and patient care.
This article underscores the necessity of familiarizing oneself with local regulations, assessing infrastructure, and grasping cultural nuances to ensure effective patient recruitment and retention. It also highlights the importance of aligning site capabilities with research objectives while maintaining open lines of communication with local stakeholders to foster collaboration and trust. Incorporating flexibility in scheduling further empowers researchers to navigate the complexities inherent in clinical trials.
In conclusion, the importance of comprehensive site feasibility planning in Serbia cannot be overstated. As the landscape of clinical research evolves, particularly with the upcoming regulatory changes in 2025, adopting these best practices will be essential. Researchers are urged to embrace these strategies, ensuring that their studies not only meet regulatory requirements but also resonate with the local communities they aim to serve.
Why is understanding local context important for site feasibility in biopharma trials in Serbia?
Understanding local context is crucial for successful clinical research, as it encompasses cultural, regulatory, and logistical factors that can impact the feasibility and execution of trials.
What should researchers familiarize themselves with regarding local regulations in Serbia?
Researchers should familiarize themselves with Serbia's clinical trial regulations, which can differ significantly from other regions, to navigate approval processes effectively and prepare for upcoming revisions in research protocols expected in 2025.
How does infrastructure affect site feasibility planning in Serbia?
Evaluating the availability and quality of local infrastructure, such as transportation networks, healthcare facilities, and communication systems, is vital for guiding location choice and ensuring that experiments can be conducted smoothly and efficiently.
What role do cultural considerations play in patient recruitment for clinical trials?
Understanding cultural differences is key for effective patient recruitment and retention. Engaging with local communities and appreciating their values can enhance trust and participation in clinical studies.
Why are logistical partnerships important in site feasibility planning?
Collaborating with local logistics providers is critical for optimizing transportation and access to research sites, ensuring that resources and staff can reach these locations promptly, which is vital for maintaining timelines and operational effectiveness.
How can integrating these components into feasibility planning impact clinical trials in Serbia?
By integrating local regulations, infrastructure assessments, cultural considerations, and logistical partnerships into the feasibility planning process, researchers can significantly boost the likelihood of successful execution of clinical trials in Serbia.