


The article delineates four essential strategies for effective clinical trial monitoring:
Each strategy is underpinned by detailed steps and practices aimed at enhancing the reliability and efficiency of clinical trials. This includes:
Such measures are crucial for addressing the complexities of clinical research, ensuring that trials are conducted with the utmost integrity and precision.
Effective clinical trial monitoring stands as a cornerstone of successful medical research, ensuring that studies adhere to safety and compliance standards while delivering reliable results. By implementing strategic approaches, research managers can significantly enhance the integrity of their trials and foster a culture of accountability among clinical teams.
However, with the ever-evolving regulatory landscape and the increasing complexity of trials, organizations must consider: how can they effectively navigate these challenges to optimize outcomes? This article delves into four key strategies that not only streamline monitoring processes but also leverage technology to elevate both efficiency and safety in clinical trials.
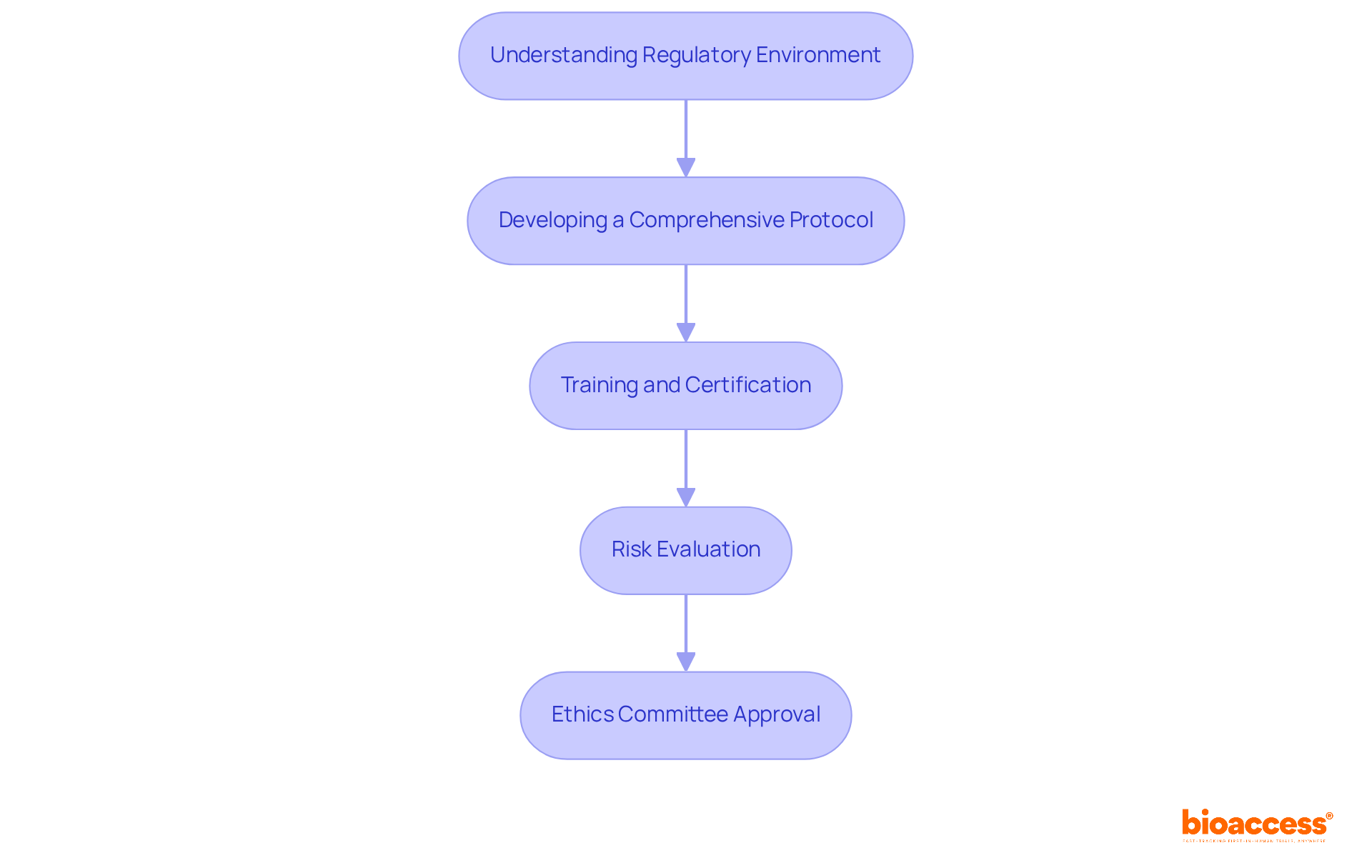
To establish adherence and security criteria in research studies, it is crucial to start with a comprehensive understanding of the regulatory environment. This includes familiarizing yourself with guidelines from organizations such as the FDA, EMA, and ICH. Key steps include:
Developing a Comprehensive Protocol: Create a detailed clinical study protocol that outlines the study's objectives, design, methodology, and statistical considerations. This document should also include plans for clinical trial monitoring and criteria for participant inclusion and exclusion.
Training and Certification: Ensure that all personnel involved in the study are adequately trained and certified in Good Clinical Practice (GCP) and relevant regulatory requirements. Regular training updates should be scheduled to keep the team informed of any changes in regulations.
Risk Evaluation: Perform a comprehensive risk evaluation to identify possible concerns and create mitigation strategies. This proactive method assists in reducing risks to participants and improves the overall security of the study.
Ethics Committee Approval: Obtain authorization from an independent ethics committee or institutional review board (IRB) before starting the study. This step is vital for ensuring that the study meets ethical standards and that participant rights are protected.
By prioritizing compliance and safety, research managers can cultivate a culture of accountability and transparency, ultimately resulting in more dependable and ethically sound research outcomes.
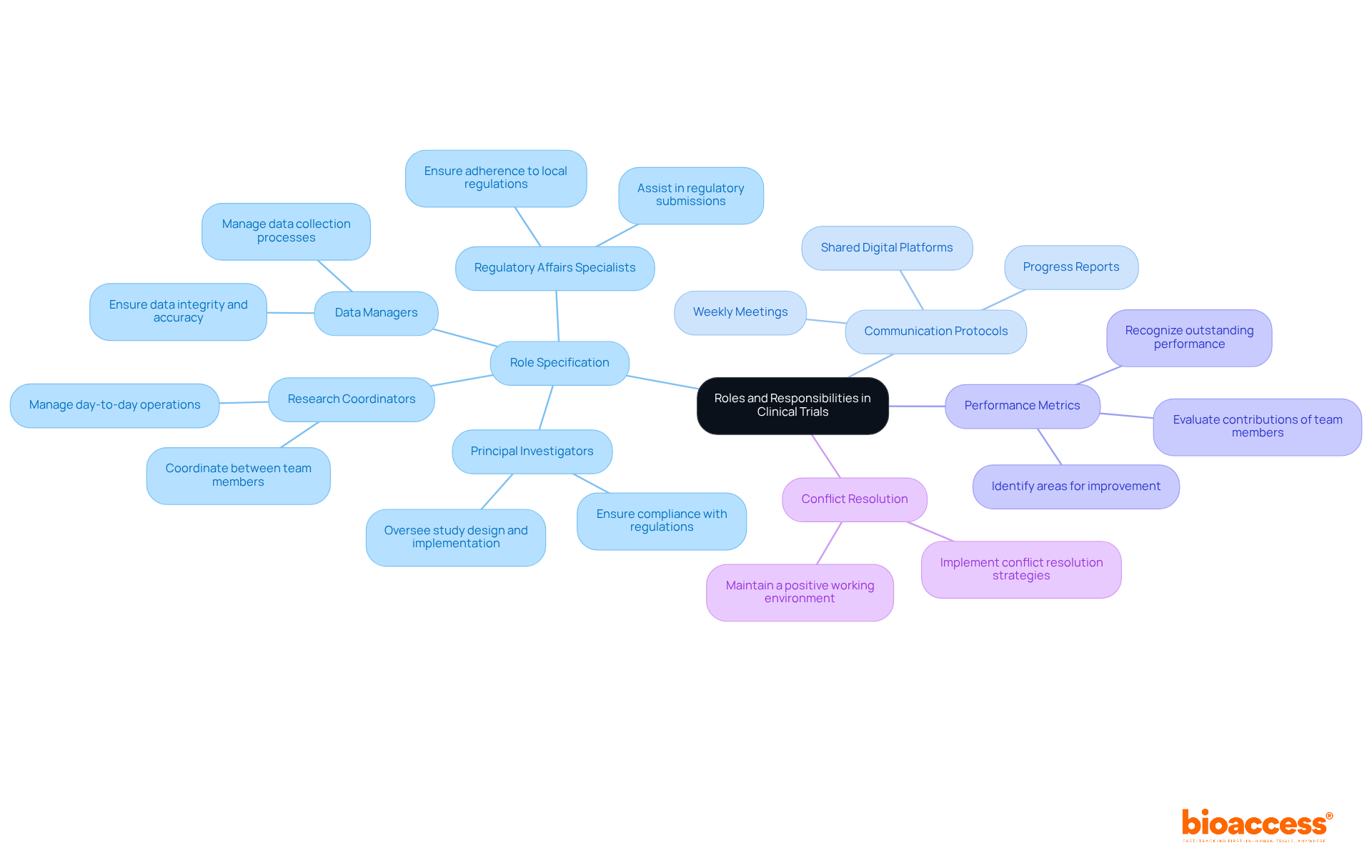
Establishing roles and responsibilities within a research team is essential for operational efficiency in clinical trial monitoring. Clearly defined roles not only streamline processes but also enhance collaboration and accountability.
Role Specification: Clearly outline the roles of each team member, including principal investigators, research coordinators, data managers, and regulatory affairs specialists. Each role should have specified responsibilities that correspond with the project's objectives. For example, Katherine Ruiz, a specialist in regulatory matters for medical devices and in vitro diagnostics, plays a vital role in ensuring adherence to local regulations, which is necessary for the success of research studies in Colombia. Additionally, incorporating bioaccess's services such as feasibility studies and site selection can further clarify these roles.
Communication Protocols: Establish communication protocols to facilitate regular updates and discussions among team members. This can include weekly meetings, progress reports, and shared digital platforms for real-time collaboration, ensuring that all team members are aligned and informed.
Performance Metrics: Develop performance metrics to evaluate the contributions of each team member. This can help in identifying areas for improvement and recognizing outstanding performance. For instance, Juan Cuya, MD, with his proficiency in epidemiology and public health, can offer valuable insights into performance assessment related to health outcomes, which is essential for project management and reporting.
Conflict Resolution: Implement a conflict resolution strategy to address any disputes or misunderstandings that may arise within the team. This ensures that issues are resolved promptly, maintaining a positive working environment.
By clearly defining roles and responsibilities and integrating bioaccess's comprehensive services into clinical trial monitoring, study managers can enhance team cohesion and ensure that all aspects of the research are executed efficiently and effectively. This ultimately contributes to the success of studies and their positive impact on local economies.
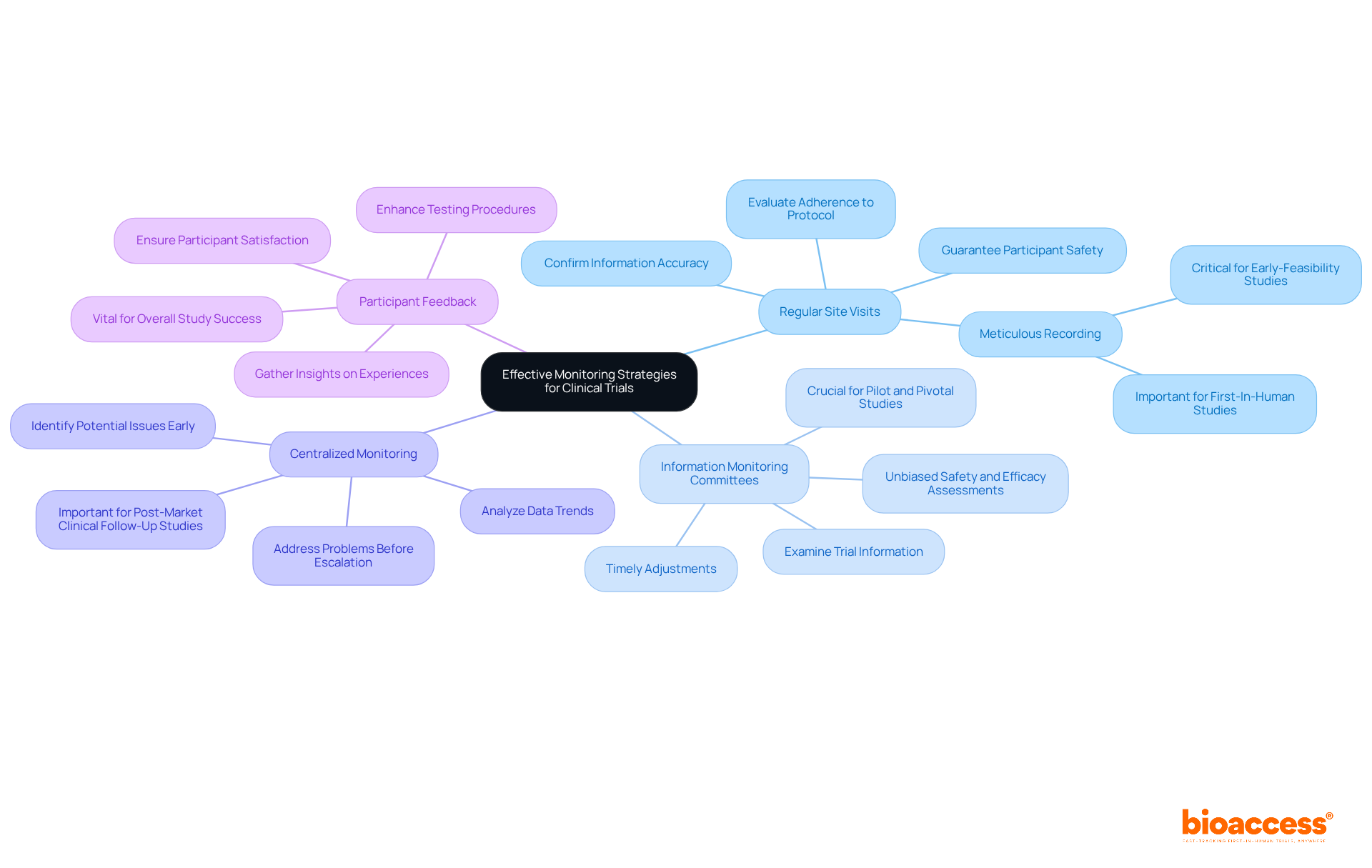
Implementing effective clinical trial monitoring strategies is essential for maintaining the integrity of clinical trials, particularly within the context of accelerated medical device clinical study services provided by bioaccess. Consider the following practices:
Regular Site Visits: Arrange routine site visits to evaluate adherence to the study protocol, confirm information accuracy, and guarantee participant safety. These visits should be recorded meticulously, especially in studies such as Early-Feasibility and First-In-Human, where preliminary information is critical.
Information Monitoring Committees: Establish autonomous information monitoring committees (DMCs) to examine trial information at predetermined intervals. DMCs offer unbiased assessments of safety and efficacy, allowing for timely adjustments if necessary—this is crucial for the success of Pilot and Pivotal studies.
Centralized Monitoring: Employ centralized monitoring techniques to analyze data trends and identify potential issues early. This method aids in addressing problems before they escalate, ensuring that the study remains on track, particularly in Post-Market Clinical Follow-Up Studies (PMCF).
Participant Feedback: Integrate participant feedback mechanisms to gather insights regarding their experiences during the study. This information is invaluable for enhancing testing procedures and ensuring participant satisfaction, which is vital for the overall success of studies managed by bioaccess.
By implementing clinical trial monitoring techniques, research managers can enhance the reliability of their studies and ensure they meet the highest standards of quality and security, leveraging the expertise of bioaccess in navigating complex research environments.
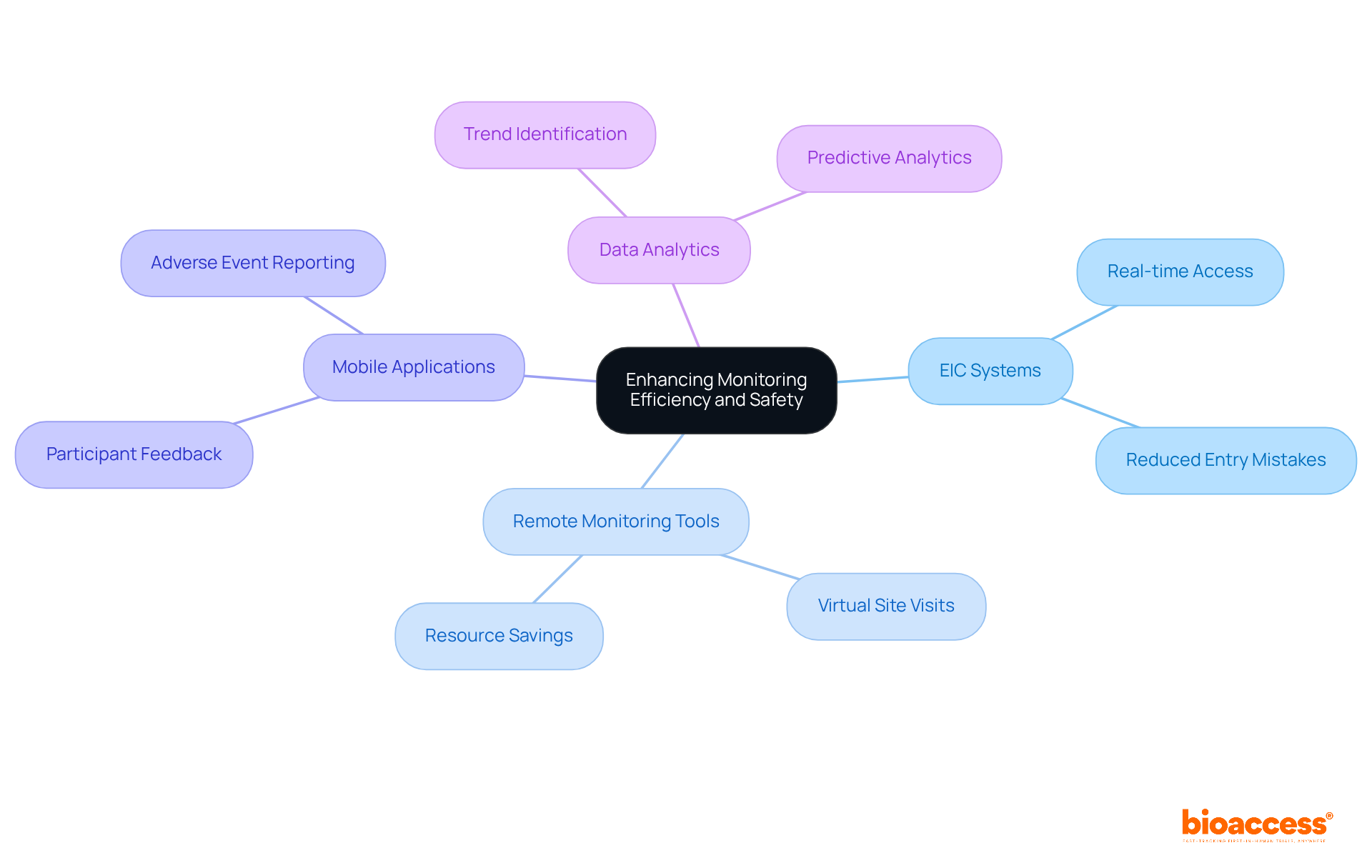
To enhance clinical trial monitoring efficiency and safety, leveraging technology is essential. Electronic Information Capture (EIC) systems should be implemented to improve the collection and management of information. These systems significantly decrease the likelihood of entry mistakes and enable real-time access for monitoring purposes.
Remote Monitoring Tools are crucial for conducting virtual site visits, allowing oversight without the need for physical presence. This strategy not only saves time and resources but also maintains essential oversight.
Additionally, developing Mobile Applications for participants enables them to report their experiences and any adverse events directly. This prompt feedback enhances participant safety and elevates the quality of information gathered.
Furthermore, utilizing advanced Data Analytics is vital for identifying trends and anomalies in experimental data. Predictive analytics can foresee potential issues, facilitating proactive management of the trial.
By integrating these technological solutions, clinical trial monitoring can be significantly enhanced in terms of efficiency and safety by clinical trial managers. This ultimately leads to more successful trial outcomes, underscoring the importance of embracing innovation in clinical research.
Establishing effective clinical trial monitoring is vital for ensuring the integrity and safety of research studies. By focusing on compliance, clearly defined roles, robust monitoring strategies, and leveraging technology, research teams can significantly enhance the outcomes of their trials. These key strategies not only promote a culture of accountability but also ensure that the rights and safety of participants are prioritized throughout the research process.
The article outlines critical steps such as:
Each of these elements plays a crucial role in mitigating risks, improving communication, and fostering collaboration among team members. Moreover, the integration of participant feedback and data analytics further enriches the monitoring process, allowing for timely interventions and adjustments.
In conclusion, the importance of effective clinical trial monitoring cannot be overstated. As the landscape of clinical research continues to evolve, embracing these best practices will be essential for achieving reliable and ethically sound outcomes. Research teams are encouraged to adopt these strategies and leverage innovative technologies to enhance their monitoring efficiency and safety, ultimately contributing to the advancement of medical research and better health outcomes for communities worldwide.
What is the first step in establishing compliance and safety standards in clinical trials?
The first step is to gain a comprehensive understanding of the regulatory environment, including guidelines from organizations such as the FDA, EMA, and ICH.
What should a comprehensive clinical study protocol include?
A comprehensive clinical study protocol should outline the study's objectives, design, methodology, statistical considerations, plans for clinical trial monitoring, and criteria for participant inclusion and exclusion.
Why is training and certification important for personnel involved in clinical trials?
Training and certification are important to ensure that all personnel are adequately informed about Good Clinical Practice (GCP) and relevant regulatory requirements, which helps maintain compliance and safety in the study.
What is the purpose of conducting a risk evaluation in clinical trials?
The purpose of conducting a risk evaluation is to identify possible concerns and create mitigation strategies, which helps reduce risks to participants and improves the overall security of the study.
Why is ethics committee approval necessary before starting a clinical trial?
Ethics committee approval is necessary to ensure that the study meets ethical standards and that participant rights are protected.
How does prioritizing compliance and safety benefit research managers?
Prioritizing compliance and safety helps research managers cultivate a culture of accountability and transparency, leading to more dependable and ethically sound research outcomes.