


Navigating the complexities of TGA GMP licensing is a critical endeavor for biopharma manufacturers. Ensuring the safety and efficacy of therapeutic products hinges on securing this vital certification. This guide unpacks the essential steps and best practices necessary for compliance, emphasizing the significance of thorough documentation.
With numerous regulatory challenges and evolving standards, how can manufacturers effectively prepare and position themselves for success in this stringent licensing landscape? This question is not just relevant; it’s imperative for those aiming to thrive in the competitive biopharma sector.
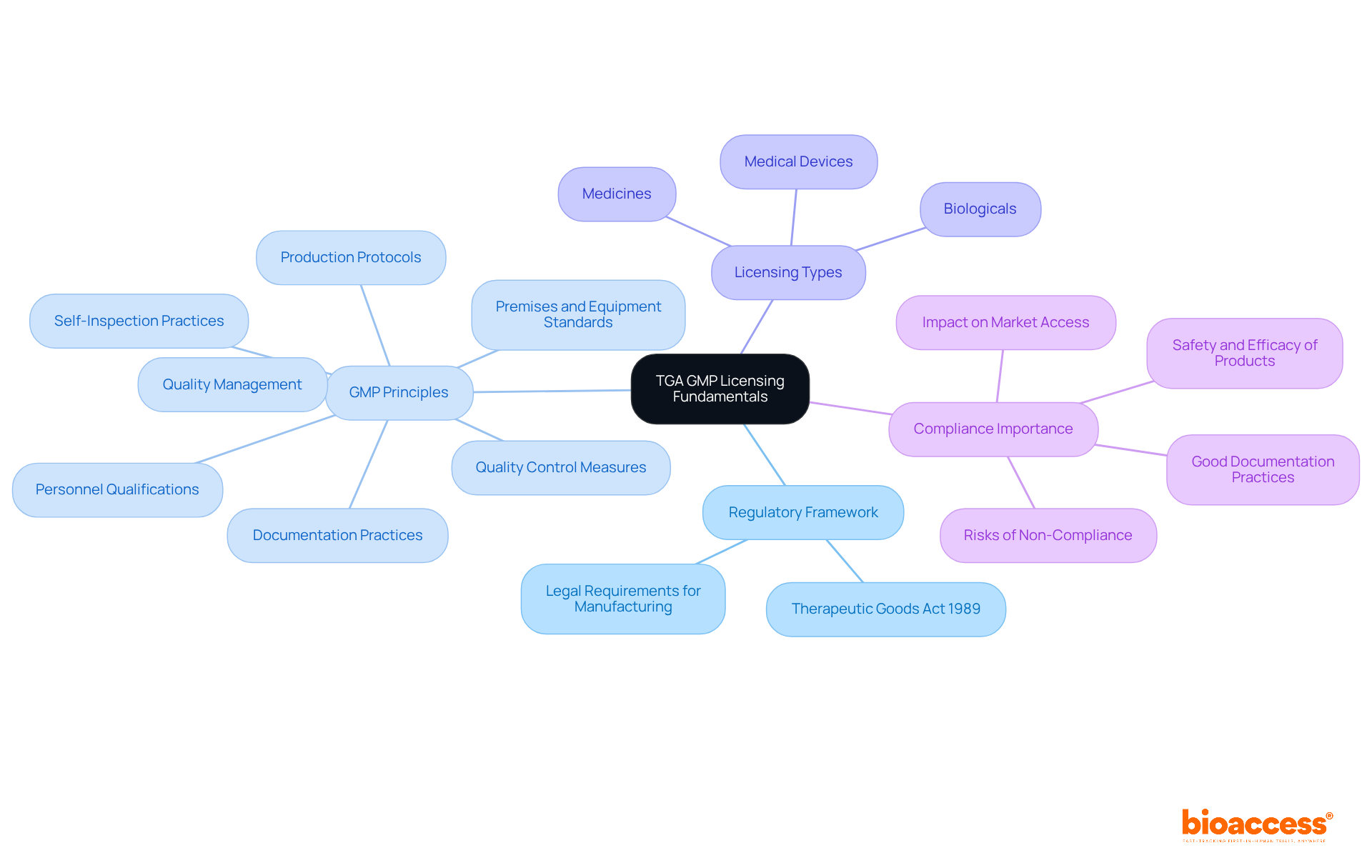
The Therapeutic Goods Administration (TGA) in Australia plays a crucial role in overseeing the production of therapeutic goods, ensuring compliance with strict quality requirements. Understanding the TGA GMP licensing for biopharma manufacturers is essential for anyone involved in clinical research, as it directly impacts the safety and efficacy of therapeutic products.
Regulatory Framework: The TGA operates under the Therapeutic Goods Act 1989, which delineates the legal requirements for manufacturing therapeutic goods in Australia. This framework ensures a robust regulatory environment that safeguards public health.
GMP Principles: Familiarizing yourself with Good Manufacturing Practice (GMP) is vital. Core principles encompass quality management, personnel qualifications, premises and equipment standards, thorough documentation, production protocols, quality control measures, and self-inspection practices. These principles are crucial for maintaining adherence and ensuring product integrity.
Licensing Types: Depending on the therapeutic goods being manufactured-whether medicines, biologicals, or medical devices-different licenses may be required. Understanding these distinctions is vital for navigating the licensing landscape effectively.
Compliance Importance: Adhering to GMP is not merely a regulatory obligation; it is fundamental to ensuring the safety and efficacy of products. This is critical for patient health and the success of biopharma ventures in the market. Good documentation practices are required throughout the product’s entire life cycle, including design stages, manufacturing and testing, batch release, warehousing and distribution, and post-marketing surveillance.
In 2025, around 32% of facilities producing registered medicines in Australia are authorized or accredited by the TGA through on-site evaluations. This statistic emphasizes the significance of adherence in sustaining market access. Recent audits have underscored the necessity for improved documentation practices, as deficiencies in compliance can lead to significant risks for patient safety and public health. By understanding these fundamentals, manufacturers can better prepare for the TGA GMP licensing for biopharma manufacturers process ahead, ensuring they meet the TGA's strict requirements.
To prepare your application for TGA GMP licensing, follow these essential steps:
Determine eligibility by confirming that your manufacturing processes and products are in alignment with the TGA GMP licensing for biopharma manufacturers. This involves ensuring your facility complies with GMP guidelines, which may require up to 15 months for international manufacturing locations to certify.
Gather Required Documentation: Compile all necessary documents, including:
Complete the Application Form: Accurately fill out the TGA's application form, ensuring all requested information is provided. Each section must be completed to prevent delays, as applications that are not fully completed will not be accepted. As the TGA states, "Ensure the nominated contact person is available throughout the evaluation process, to respond to any questions the TGA may have."
Submit Application via TGA Business Services: Utilize the TGA Business Services (TBS) portal for submission. Ensure that you pay any applicable fees and attach all required documents. The average duration for typical applications usually takes about 255 working days, but with adequate preparation, you can optimize this process. The TGA increasingly relies on FDA and EMA reviews to expedite approvals, which can also impact your application timeline.
By meticulously preparing your application and adhering to these guidelines, you significantly enhance your chances of a smooth approval process for TGA GMP licensing for biopharma manufacturers.

To ensure compliance and uphold documentation standards in clinical research, manufacturers must adopt effective practices:
Implement a Quality Management System (QMS): Establishing a robust QMS is essential. It should clearly outline procedures for quality assurance, control, and continuous improvement. Regular evaluations and revisions of this system are crucial to adapt to evolving criteria.
Maintain Accurate Records: Keeping detailed records of all manufacturing processes, quality control tests, and staff training is vital. These documents must be organized and readily accessible for audits and inspections. Accurate record-keeping is essential for adhering to GMP regulations. Currently, only 62% of organizations achieve complete compliance with documentation requirements, highlighting the need for improved methods.
Conduct Internal Audits: Regular internal audits are necessary to assess compliance with GMP guidelines and identify areas for improvement. Promptly addressing any non-conformities can significantly reduce the risk of regulatory issues. Notably, a 40% decrease in adherence errors has been observed with role-specific qualification programs, underscoring the importance of tailored training in maintaining precise records.
Stay informed on regulatory changes: It is critical to keep abreast of updates to TGA GMP licensing for biopharma manufacturers and related guidelines. As these documentation guidelines evolve, manufacturers must proactively adapt their practices to remain compliant. This proactive approach is essential in the rapidly expanding biologics market, projected to grow at a compound annual growth rate of 15% until 2027.
By prioritizing compliance and meticulous documentation, manufacturers can establish a solid foundation for TGA GMP licensing for biopharma manufacturers. This commitment ultimately enhances operational efficiency and market competitiveness.

Obtaining TGA GMP licensing presents several common challenges that manufacturers must navigate effectively:
Incomplete Applications: Applications often face rejection due to missing information or documents. In fact, a significant percentage of applications are rejected due to non-compliance with GMP regulations, emphasizing the importance of thoroughness.
Non-Compliance Issues: Inspections frequently uncover non-compliance with GMP standards, resulting in delays or outright rejections. According to the FDA, it is essential for manufacturers to establish a quality culture that prioritizes adherence to regulations.
Communication Gaps: Misunderstandings with TGA representatives can lead to confusion regarding application requirements.
Regulatory Changes: Evolving TGA regulations can significantly affect the licensing process.
By addressing these challenges proactively, manufacturers can significantly improve their likelihood of securing TGA GMP licensing for biopharma manufacturers.

Understanding the TGA GMP licensing process is crucial for biopharma manufacturers who aim to ensure the safety and efficacy of their therapeutic products. This guide outlines the essential steps involved in obtaining TGA GMP licensing, underscoring the importance of compliance with the stringent regulatory standards set by the Therapeutic Goods Administration in Australia.
Key points discussed include:
Furthermore, preparing a comprehensive application, meticulous documentation, and strict adherence to compliance standards are vital for a successful licensing process. Manufacturers must proactively address common challenges, such as incomplete applications and non-compliance issues, to enhance their chances of approval.
Ultimately, prioritizing TGA GMP licensing not only upholds public health standards but also positions biopharma manufacturers competitively in the market. By committing to rigorous documentation practices and staying informed on regulatory changes, manufacturers can navigate the licensing landscape effectively. This ensures that their products meet the highest quality standards and contribute positively to patient health.
What is the role of the Therapeutic Goods Administration (TGA) in Australia?
The TGA oversees the production of therapeutic goods in Australia, ensuring compliance with strict quality requirements to safeguard public health.
What is the regulatory framework under which the TGA operates?
The TGA operates under the Therapeutic Goods Act 1989, which outlines the legal requirements for manufacturing therapeutic goods in Australia.
Why is it important to understand Good Manufacturing Practice (GMP)?
Understanding GMP is vital as it encompasses core principles such as quality management, personnel qualifications, premises and equipment standards, documentation, production protocols, quality control measures, and self-inspection practices, all crucial for maintaining product integrity.
What types of licenses are required for manufacturing therapeutic goods?
Different licenses may be required depending on the type of therapeutic goods being manufactured, including medicines, biologicals, or medical devices.
Why is compliance with GMP important?
Compliance with GMP is essential not only as a regulatory obligation but also for ensuring the safety and efficacy of products, which is critical for patient health and the success of biopharma ventures.
What documentation practices are necessary throughout the product lifecycle?
Good documentation practices are required throughout the entire lifecycle of the product, including design, manufacturing and testing, batch release, warehousing and distribution, and post-marketing surveillance.
What percentage of facilities producing registered medicines in Australia are authorized or accredited by the TGA?
In 2025, around 32% of facilities producing registered medicines in Australia are expected to be authorized or accredited by the TGA through on-site evaluations.
What has recent auditing revealed about compliance?
Recent audits have highlighted the need for improved documentation practices, as deficiencies in compliance can pose significant risks to patient safety and public health.