


To achieve MDR 2017/745 compliance for Medtech, organizations must follow four critical steps:
Understanding these steps is essential for organizations aiming to mitigate delays and ensure market readiness. With the compliance deadline approaching in May 2024, thorough preparation is not just advisable—it is imperative for success in the Medtech landscape.
Navigating the complex landscape of medical device regulations is no small feat, particularly with the stringent requirements set forth by the Medical Device Regulation (MDR) 2017/745. As the deadline for compliance looms closer, understanding the critical steps necessary for adherence becomes paramount for manufacturers aiming to ensure their products meet safety and performance standards in the European market.
However, with numerous challenges—including intricate documentation, resource limitations, and evolving regulatory landscapes—how can organizations effectively position themselves for success?
This guide outlines essential strategies and actionable steps to achieve MDR compliance, empowering medtech companies to overcome obstacles and thrive in a competitive environment.
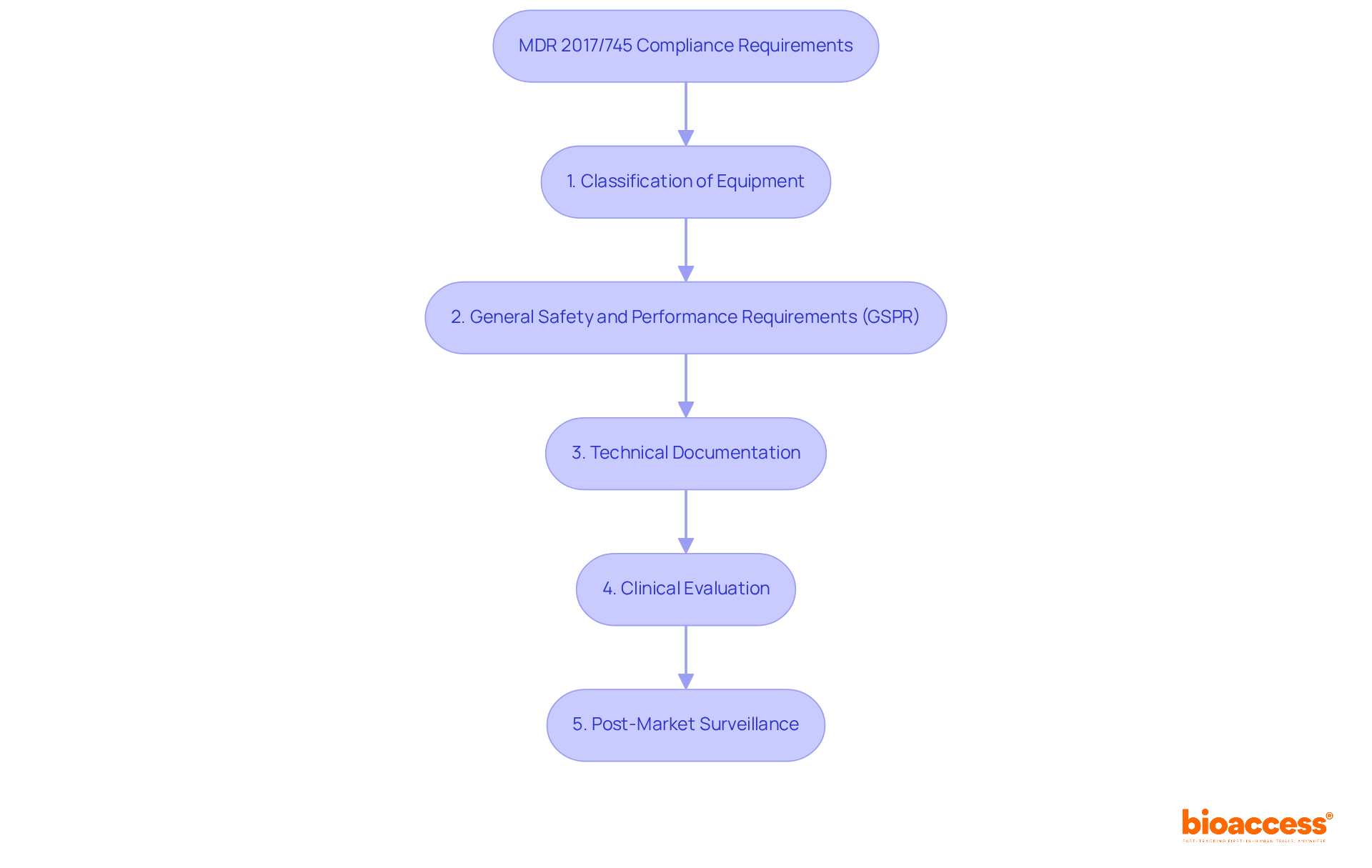
Attaining adherence to 2017/745 necessitates a comprehensive understanding of its essential prerequisites, designed to ensure the safety and effectiveness of medical products within the European market. Key components include:
Classification of Equipment: Accurately categorize your medical apparatus based on its intended use, risk level, and duration of body contact. This classification is crucial as it determines the regulatory pathway and specific requirements that must be met.
General Safety and Performance Requirements (GSPR): Familiarize yourself with the GSPR detailed in Annex I of the regulation. These criteria specify the safety and performance benchmarks that products must meet to achieve market entry.
Technical Documentation: Compile comprehensive technical documentation that demonstrates adherence to the GSPR. This should encompass aspects such as design specifications, manufacturing processes, and a robust risk management plan.
Clinical Evaluation: Conduct a thorough clinical evaluation to assess the safety and performance of your product. This assessment is vital, ensuring that the apparatus meets the required health and safety standards.
Post-Market Surveillance: Develop a post-market surveillance strategy to continuously monitor the device's performance after it enters the market. This ongoing evaluation is essential for upholding regulations and ensuring user safety.
Understanding these requirements empowers entities to efficiently plan their adherence initiatives, thereby reducing the risk of delays or violations as the sector encounters anticipated bottlenecks in the coming years. As of September 2021, only 502 conformity assessment certificates had been granted, underscoring the urgency for manufacturers to act swiftly to meet the full adherence deadline of 2017/745 by May 2024. bioaccess® provides extensive clinical trial management services, including feasibility studies, site selection, regulatory reviews, trial setup, import permits, project management, and reporting, to support entities in navigating these complexities. With Katherine Ruiz's expertise in regulatory matters for medical devices and in vitro diagnostics in Colombia, bioaccess® is well-equipped to assist Medtech, Biopharma, and Radiopharma startups in meeting their requirements.
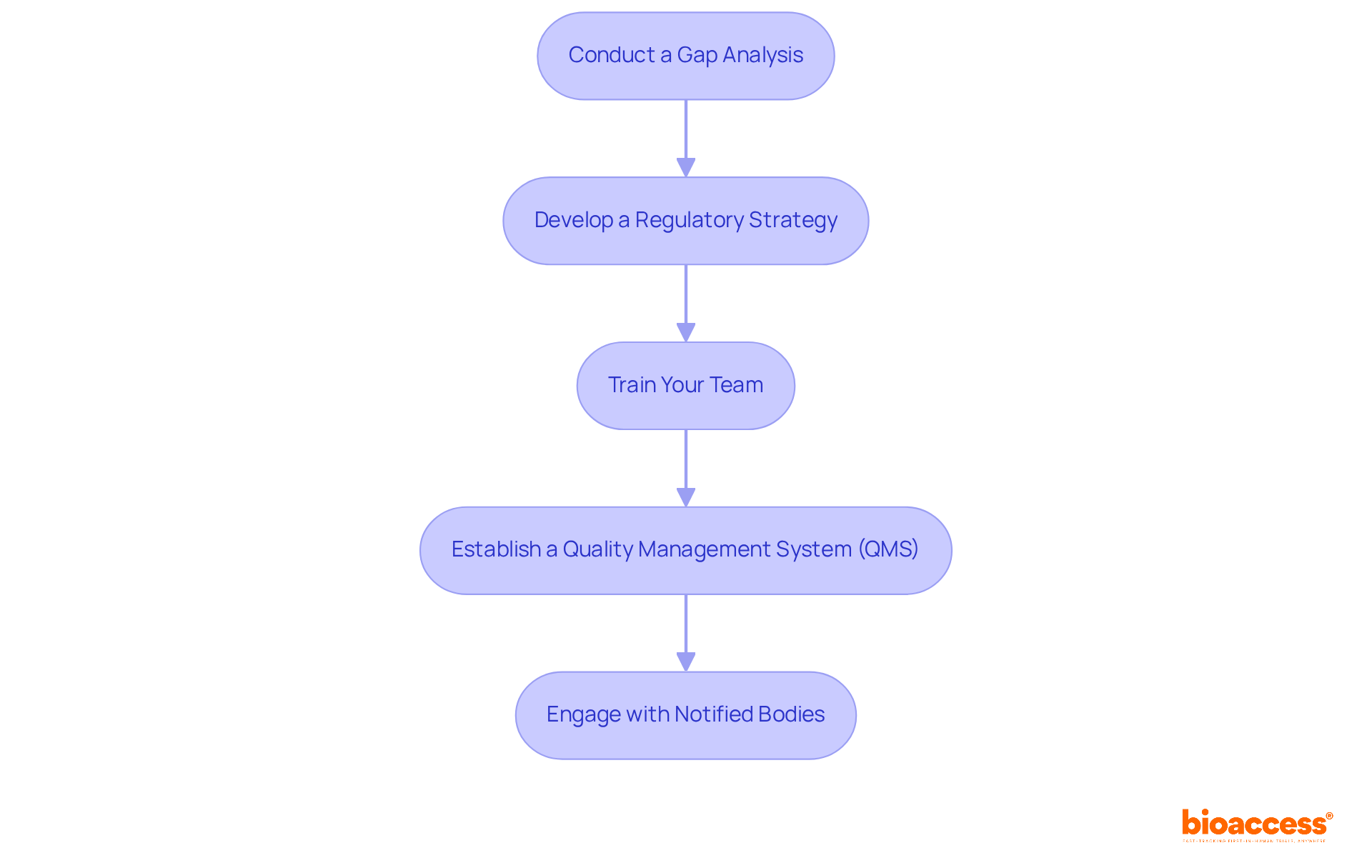
Preparing your organization for MDR compliance involves several critical steps:
Conduct a Gap Analysis: Begin by evaluating your existing processes, documentation, and adherence status against the MDR requirements. This analysis is essential for identifying areas needing improvement or additional resources. Regular gap analyses are recommended, especially in light of the transition from the Medical Device Directive (MDD) to 2017/745, as many MDD-compliant technical files may not meet the new standards. It is advisable to conduct a gap analysis annually before the Management Review to prevent regulatory and adherence issues.
Develop a Regulatory Strategy: Formulate a comprehensive plan that outlines how your organization will achieve adherence. This strategy should encompass timelines, responsibilities, and resource distribution, ensuring that all elements of adherence are addressed systematically.
Train Your Team: It is essential to ensure that all pertinent staff members are knowledgeable about MDR requirements and comprehend their roles in the adherence process. Training can take various forms, including workshops, seminars, or online courses, to enhance knowledge and preparedness.
Establish a Quality Management System (QMS): Implement or update your QMS to align with MDR standards. This system should record and manage all processes associated with product development, manufacturing, and post-market activities, ensuring adherence and operational efficiency. As Anne Holland, CEO and Founder, states, "A medical device gap analysis evaluates your current Quality Management System (QMS) compared to industry standards and regulations to determine if any gaps exist between them."
Engage with Notified Bodies: Initiate discussions with Notified Bodies early in the regulatory journey. Understanding their requirements and timelines can significantly streamline the certification process, reducing the risk of delays.
By adhering to these preparatory steps, organizations can effectively position themselves for a successful regulatory journey, minimizing risks and enhancing their operational readiness. For instance, a case study demonstrated that a European medical equipment manufacturer made significant progress on their EU MDR compliance project by conducting a thorough gap assessment, allowing them to shift focus to Clinical Evaluation Report (CER) work.
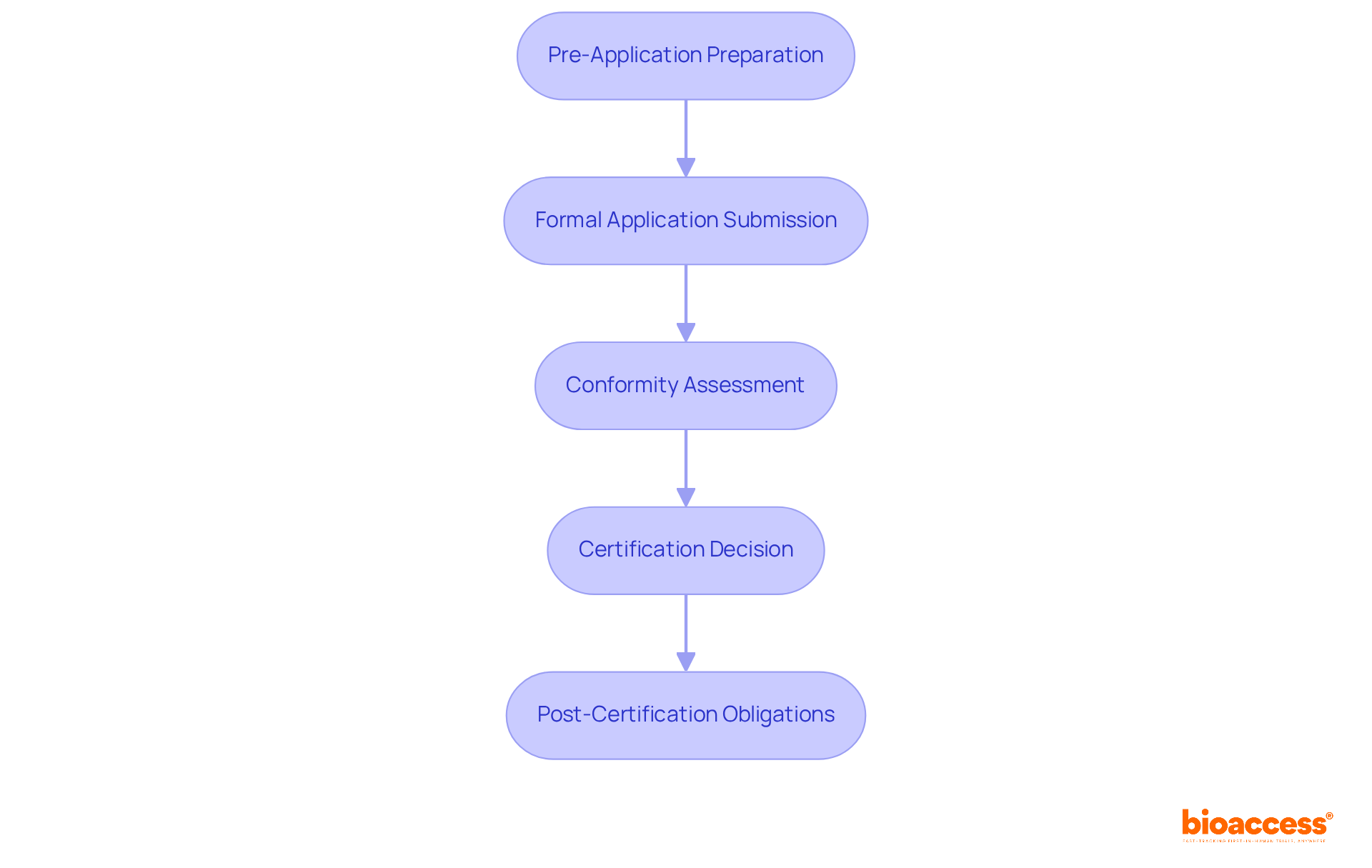
The MDR certification process encompasses several critical stages that are essential for successful navigation:
Pre-Application Preparation: Before submitting your application, it is imperative to ensure that all technical documentation is complete and your Quality Management System (QMS) is firmly established. This preparation includes the Medical Device Questionnaire and any necessary supporting documents. Manufacturers are strongly encouraged to maintain high documentation quality, as poor quality has historically posed significant challenges in past applications.
Formal Application Submission: The next step involves submitting your application to the selected Notified Body, ensuring that all required documentation is included. A thorough submission is crucial to minimize delays; incomplete application files have been a prominent reason for rejection, with 176 applications rejected in October 2022 alone.
Conformity Assessment: Following submission, the Notified Body will conduct a conformity assessment, which may include audits of your QMS and reviews of your technical documentation. Be prepared to provide additional information or clarifications as requested. The re-certification timeline typically spans about 12 months, influenced by the classification and complexity of the application.
Certification Decision: If your product aligns with all regulatory requirements, the Notified Body will issue a CE certificate, thus enabling you to market your product within the EU. Conversely, if your application does not meet the necessary standards, you will receive detailed feedback on areas that require improvement, allowing for resubmission after addressing the identified deficiencies.
Post-Certification Obligations: Once certification is achieved, it is vital to uphold standards by adhering to post-market surveillance requirements. Additionally, any modifications to the equipment or processes must be communicated to the Notified Body to avoid delays in certification renewal.
By diligently adhering to these stages, companies can effectively navigate the MDR certification process and achieve compliance, ultimately facilitating the successful introduction of their medical devices to the European market.
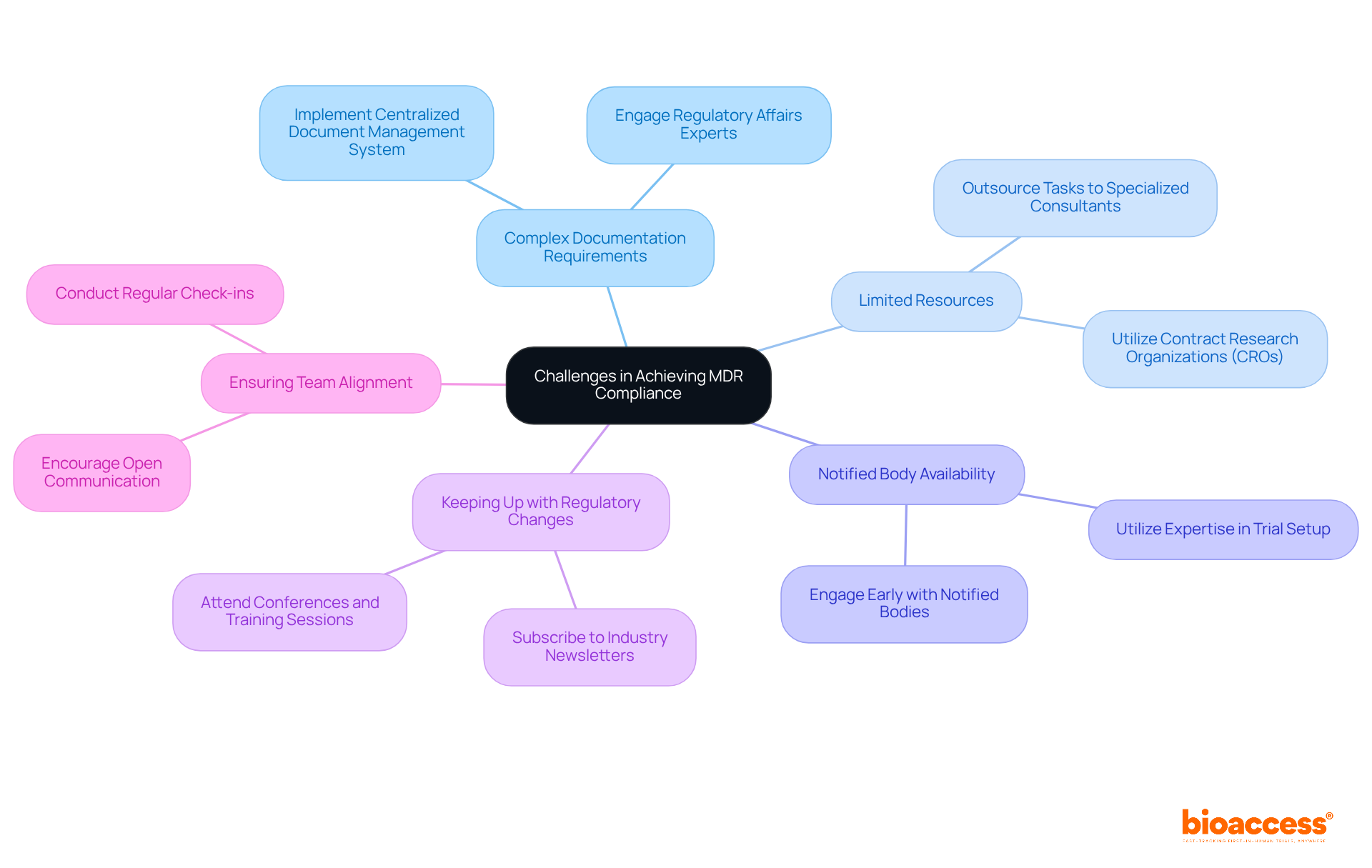
Achieving compliance with the Medical Device Regulation (MDR) presents numerous challenges that organizations must navigate effectively. Here are some common obstacles and effective strategies to address them:
Complex Documentation Requirements: The extensive documentation mandated by the MDR can be daunting. Implementing a centralized document management system is crucial for organizing all necessary documents and tracking revisions efficiently. This approach not only streamlines the documentation process but also enhances data integrity, reducing the risk of errors. Engaging with experts in regulatory affairs, such as Katherine Ruiz, can provide valuable insights into navigating these complexities.
Limited Resources: Resource constraints, including time and personnel, are prevalent in many entities. To relieve this pressure, consider outsourcing particular tasks to specialized consultants or contract research firms (CROs) like bioaccess. Their extensive clinical trial management services, including feasibility studies, site selection, and regulatory reviews, enable internal teams to concentrate on essential activities while ensuring that regulatory tasks are managed by specialists. According to industry insights, numerous entities face resource limitations that can hinder compliance efforts.
Navigating Notified Body Availability: With numerous manufacturers vying for certification, the availability of Notified Bodies can be limited. Engaging with these bodies early in the process is essential to secure timelines and gain a clear understanding of their requirements. Early communication can facilitate smoother interactions and help avoid delays. Utilizing bioaccess's expertise in trial setup and project management can further enhance these interactions.
Keeping Up with Regulatory Changes: The regulatory landscape is continuously evolving, making it imperative to stay informed. Subscribing to industry newsletters, attending conferences, and participating in relevant training sessions can provide valuable insights into the latest regulatory updates and best practices. The European Commission's intentions to amend the MDR and IVDR regulations further highlight the necessity for entities to stay alert. Leveraging the knowledge of regulatory experts can help organizations adapt to these changes effectively.
Ensuring Team Alignment: Misalignment among team members can create adherence gaps. To promote alignment, encourage open communication and conduct regular check-ins to ensure that everyone is on the same page regarding regulatory objectives and timelines. This collaborative method can significantly improve the effectiveness of adherence efforts. As Paul Koziarz highlights, entities must continually push themselves to stay fully aligned with regulations and find methods to surpass benchmarks.
By proactively addressing these challenges and utilizing comprehensive clinical trial management services, including feasibility studies and site selection, organizations can significantly improve their chances of achieving MDR compliance successfully, ultimately leading to enhanced product safety and market access.
Achieving compliance with MDR 2017/745 transcends mere regulatory obligation; it is a fundamental aspect of ensuring the safety and effectiveness of medical devices within the European market. By comprehensively understanding the key requirements, organizations can strategically navigate the complexities involved in meeting these standards, ultimately positioning themselves for success in a competitive landscape.
This article outlines the essential steps for compliance, including:
Furthermore, it emphasizes the critical importance of preparing organizations through:
These measures are indispensable for overcoming the challenges posed by complex documentation requirements, limited resources, and evolving regulations.
As the deadline for full compliance approaches, proactive engagement with the MDR requirements is vital for manufacturers. By leveraging expert resources and maintaining open communication within teams, organizations can enhance their operational readiness and streamline the certification process. Ultimately, embracing these strategies not only facilitates compliance but also fosters a culture of safety and quality, ensuring that medical devices meet the highest standards for patient care.
What is the purpose of the MDR 2017/745 regulation?
The MDR 2017/745 regulation aims to ensure the safety and effectiveness of medical products within the European market.
What are the key components of compliance with MDR 2017/745?
The key components include classification of equipment, General Safety and Performance Requirements (GSPR), technical documentation, clinical evaluation, and post-market surveillance.
How should medical devices be classified under MDR 2017/745?
Medical devices should be classified based on their intended use, risk level, and duration of body contact, which determines the regulatory pathway and specific requirements.
What do the General Safety and Performance Requirements (GSPR) entail?
The GSPR, detailed in Annex I of the regulation, specifies the safety and performance benchmarks that medical products must meet for market entry.
What is required for technical documentation under MDR 2017/745?
Technical documentation must demonstrate adherence to the GSPR and include design specifications, manufacturing processes, and a robust risk management plan.
Why is clinical evaluation important in the compliance process?
Clinical evaluation is vital to assess the safety and performance of the product, ensuring it meets the required health and safety standards.
What is the role of post-market surveillance in MDR compliance?
Post-market surveillance involves developing a strategy to continuously monitor the device's performance after it enters the market, which is essential for upholding regulations and ensuring user safety.
What is the urgency for manufacturers regarding MDR 2017/745 compliance?
As of September 2021, only 502 conformity assessment certificates had been granted, highlighting the urgency for manufacturers to meet the full adherence deadline of May 2024.
How can bioaccess® assist with MDR 2017/745 compliance?
Bioaccess® provides extensive clinical trial management services, including regulatory reviews and project management, to support entities in navigating the complexities of compliance with MDR 2017/745.
MDR 2017/745 (GSPR) quotes the concept of reducing risk "as far as possible" several times.
→ Demonstrating that we have done… | EU MDR Compliance | 34 comments (https://linkedin.com/posts/eu-mdr-compliance-b15028260_how-can-risk-be-reduced-as-far-as-possible-activity-7196388834585051137-smAk)