


Navigating the complex landscape of FDA 510(k) approvals is a critical endeavor for medical device manufacturers aiming to bring innovative products to market. This regulatory pathway not only facilitates faster access to essential medical technologies but also demands a thorough understanding of its intricate requirements. With a staggering 75% of submissions facing rejection on the first attempt, the stakes are high. How can companies ensure their applications stand out in a crowded field?
This guide outlines four essential steps to successfully maneuver through the 510(k) process, equipping manufacturers with the knowledge to enhance their chances of approval and ultimately improve patient care.
The process of FDA 510k approvals serves as a vital premarket submission, allowing manufacturers to demonstrate that a new medical product is substantially equivalent to an already legally marketed product. This process is essential for manufacturers looking to navigate the regulatory landscape effectively. Its significance lies in enabling faster market access for products that meet established safety and efficacy standards, ultimately benefiting both patients and healthcare providers.
Understanding the standards for substantial equivalence is crucial, as it requires the new product to be as safe and effective as its predicate. This pathway primarily applies to Class II devices, categorized as moderate-risk. Successfully navigating the 510(k) process can significantly reduce time to market, enhancing a company's competitive advantage. For instance, the typical duration for FDA 510K approvals is around 33 months, with some applications taking as few as 2 months, while others may extend to 132 months. Notably, 85% of 510(k) applications receive a Substantially Equivalent decision from the FDA, underscoring the effectiveness of FDA 510k approvals. However, it is important to recognize that 75% of entries are rejected on the first attempt, highlighting the necessity for thorough preparation.
Additionally, the average cost for companies to achieve FDA 510K approvals is approximately $6.1 million, reflecting the financial implications of the process. Furthermore, the FDA's eSTAR requirement, which became mandatory in October 2023, is a recent update that manufacturers must consider. This variability emphasizes the importance of comprehensive preparation and a solid understanding of regulatory requirements to ensure a successful filing.
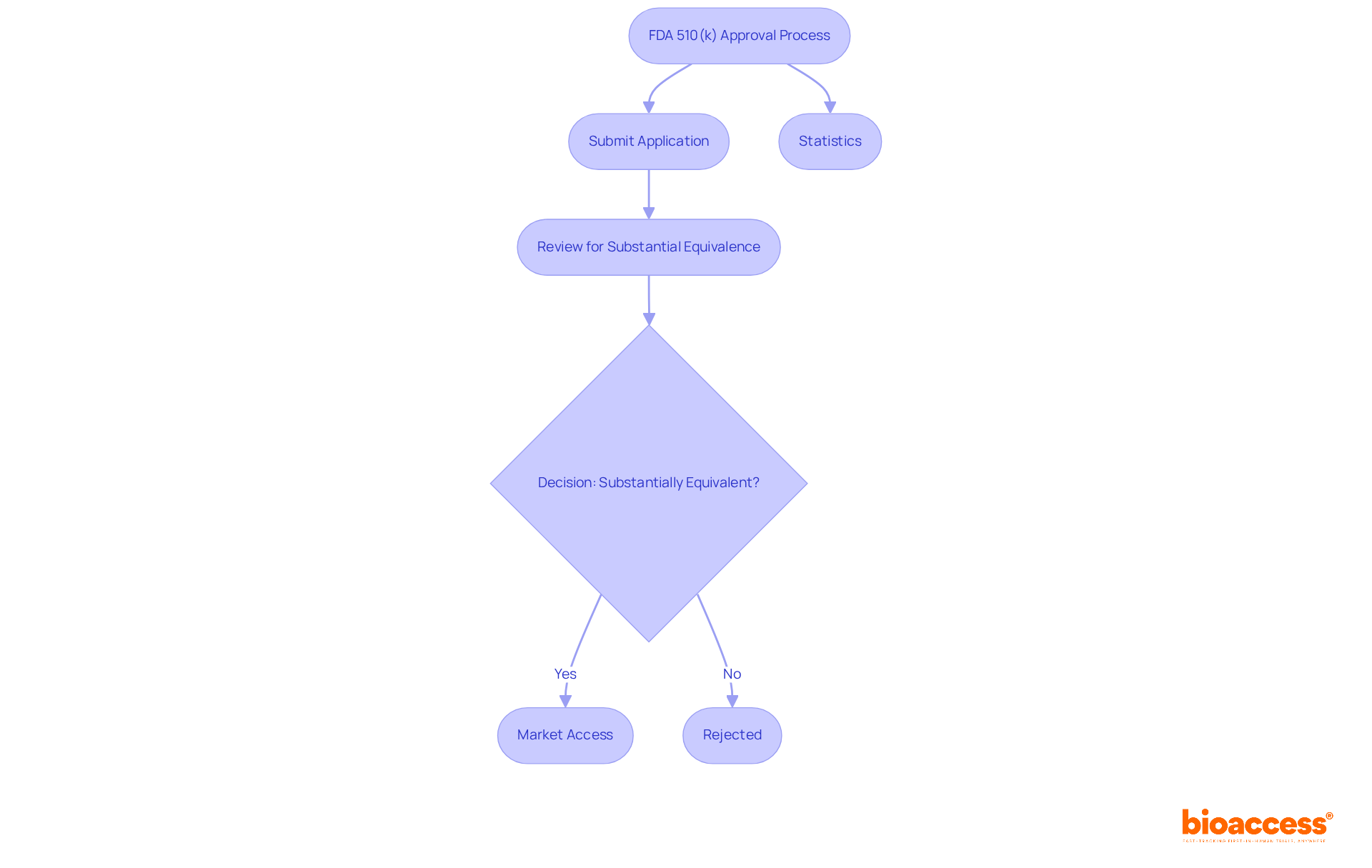
Successfully navigating the FDA 510k approvals application process is crucial for ensuring compliance with FDA regulations. Understanding these requirements not only streamlines your submission but also enhances the safety and effectiveness of medical devices.
Key Steps to Consider:
For the year ending September 2022, 67 percent of applications for FDA 510K approvals resulted in requests for additional information during the review process. This statistic underscores the importance of thorough documentation and clarity in your application. Collaborating with regulatory advisors can provide valuable insights into classification and help you avoid common pitfalls that lead to approval rejections. By proactively addressing these regulatory challenges, you can enhance your commitment to delivering safe and effective medical devices.
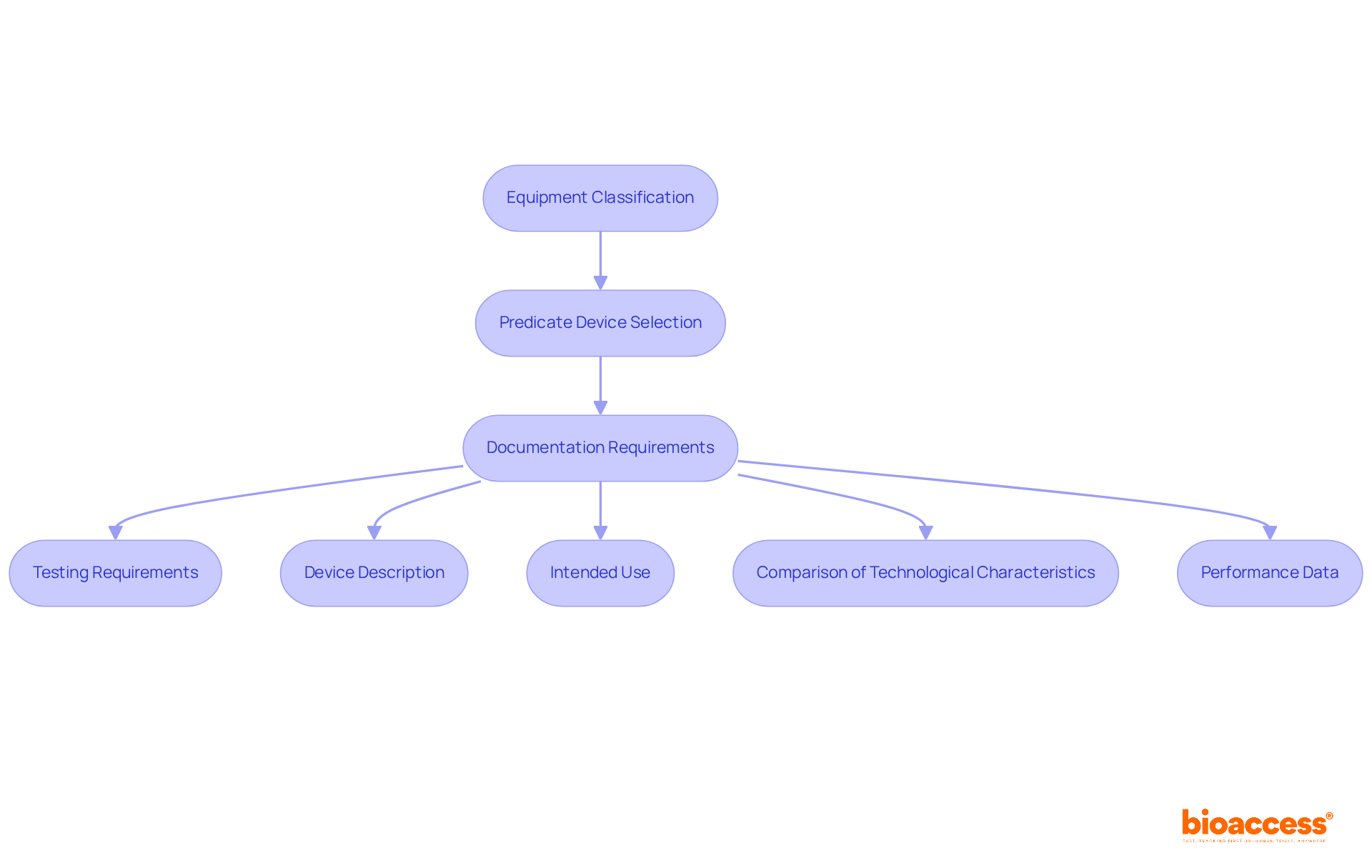
When clinical data is necessary for a 510(k) application, it is essential to design and conduct studies that adhere to FDA guidelines to achieve FDA 510k approvals. Here are the steps to follow:
Tips:
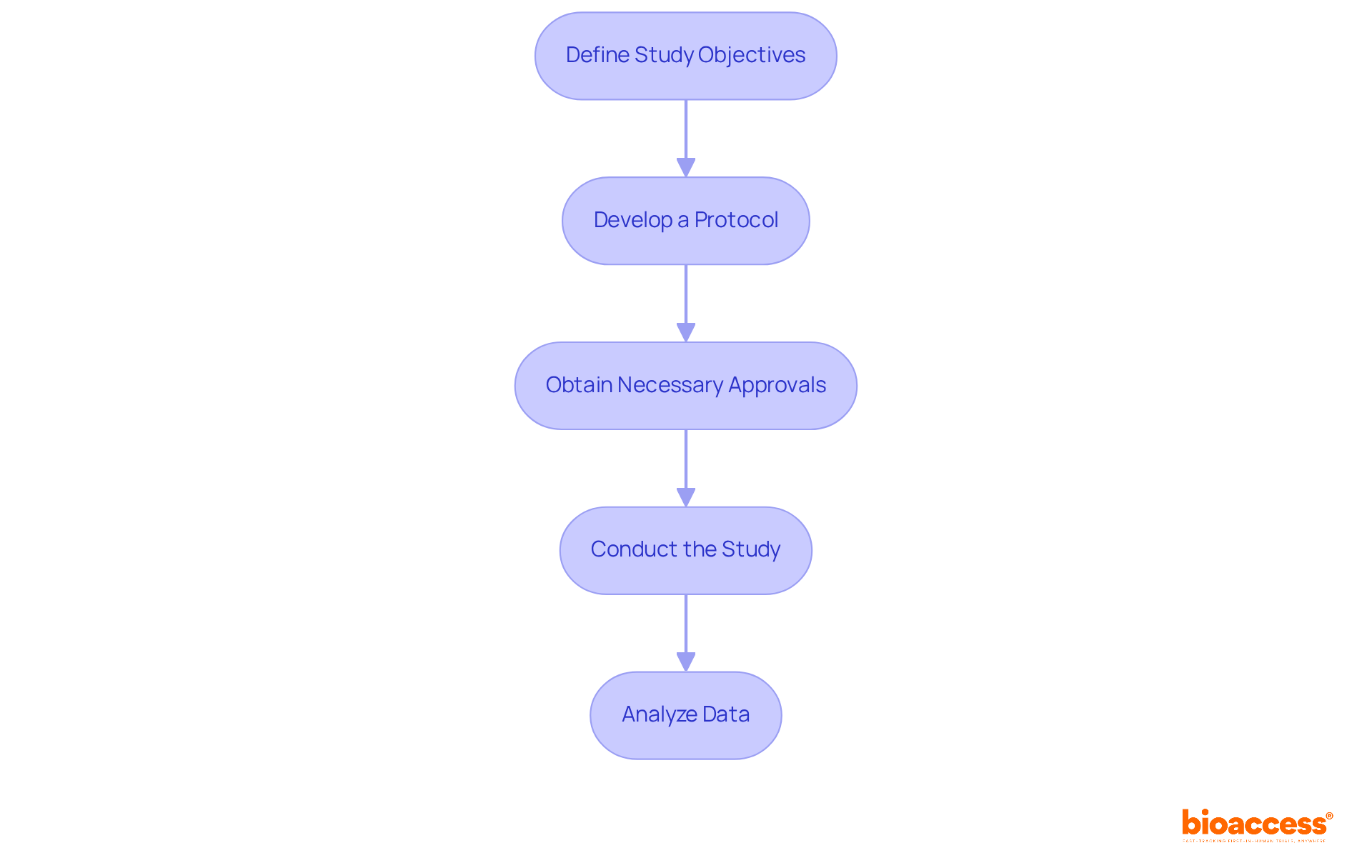
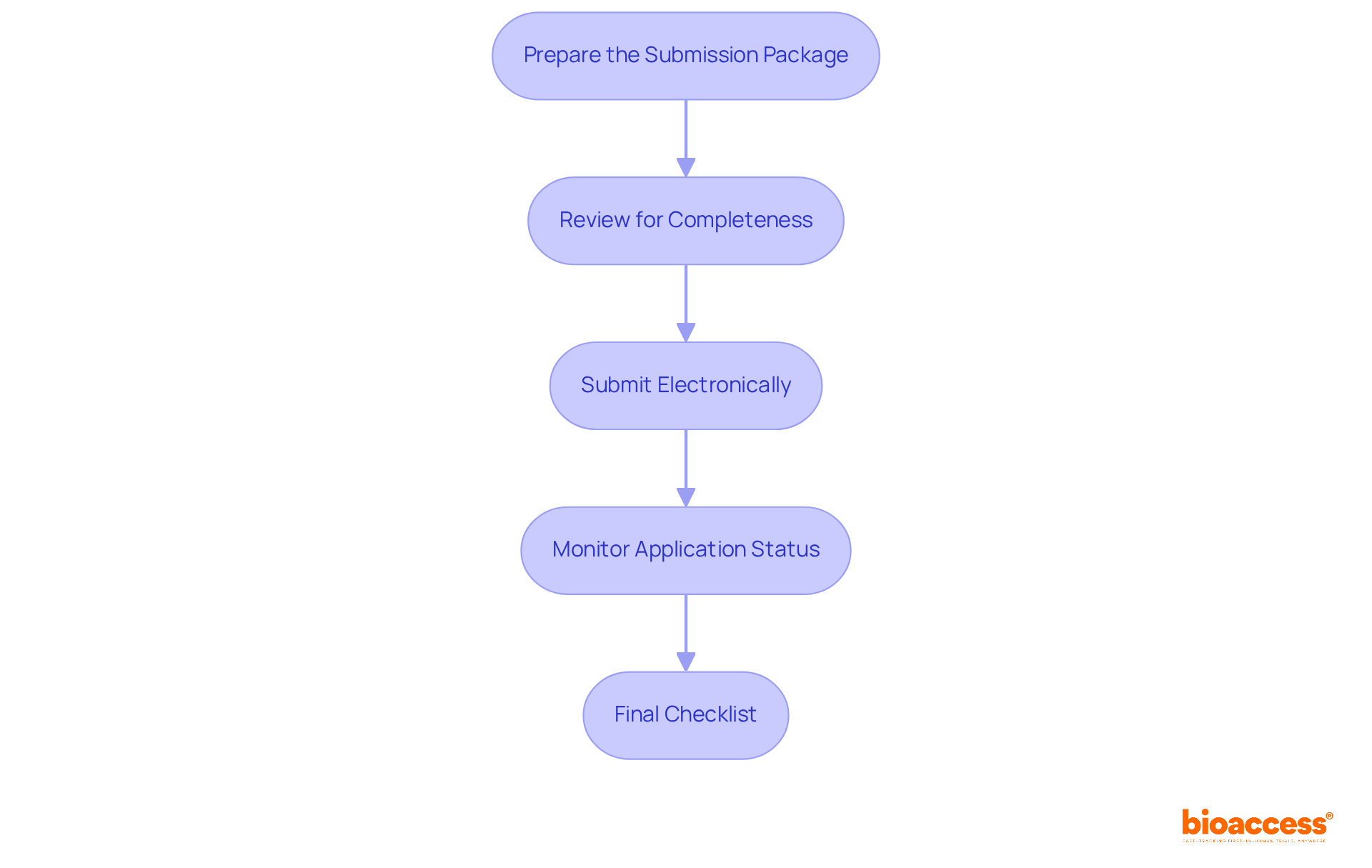
Once you have gathered all necessary information and completed your clinical studies, the next step is to compile and submit your documentation for FDA 510k approvals. Follow these steps:
Prepare the Submission Package: Ensure your package includes:
Review for Completeness: Double-check that all required documents are included and that they meet FDA formatting guidelines. A thoroughly prepared document can significantly decrease the overall clearance time by minimizing requests for additional information, which frequently result in delays. Studies indicate that the average time for FDA 510K approvals is approximately 177 days, with only 19% of devices cleared within three months.
Submit Electronically: Utilize the FDA’s eSTAR or eCopy methods to send your 510(k) application. The FDA usually examines complete and precise filings for FDA 510k approvals within an average of 90 days, but this process can extend to 3 to 6 months depending on the complexity and quality of the material.
Monitor Application Status: After sending, track the status of your application through the FDA’s online portal. Staying informed about your entry can help you respond promptly to any requests from the FDA, which is essential for maintaining the approval timeline. As noted by industry experts, partnering with an experienced clinical trial management service like bioaccess can minimize delays and streamline the process, particularly in areas such as trial setup and compliance reviews.
Final Checklist:
Successfully navigating the FDA 510(k) approval process is essential for manufacturers looking to efficiently bring new medical devices to market. This pathway not only accelerates market access but also guarantees that products meet critical safety and efficacy standards. By grasping the intricacies of the 510(k) process, manufacturers can significantly boost their chances of success while reducing potential setbacks.
Key insights from the article emphasize the necessity of thorough preparation. Understanding regulatory requirements, selecting suitable predicate devices, and designing robust clinical studies are all vital components. Statistics reveal the challenges inherent in this process, including the high rate of initial rejections and the financial implications tied to achieving approval. By adhering to best practices and utilizing resources like regulatory advisors, manufacturers can navigate this complex landscape more effectively.
As the medical device industry evolves, staying updated on changes to the FDA 510(k) process is crucial. Companies should prioritize comprehensive documentation and proactive engagement with the FDA to streamline their submissions. Embracing these strategies not only supports compliance but also reinforces a commitment to delivering safe and effective medical devices that ultimately benefit patients and healthcare providers alike.
What is the FDA 510(k) process?
The FDA 510(k) process is a premarket submission that allows manufacturers to demonstrate that a new medical product is substantially equivalent to an already legally marketed product.
Why is the 510(k) process important?
The 510(k) process is important because it enables faster market access for medical products that meet established safety and efficacy standards, benefiting both patients and healthcare providers.
What does "substantial equivalence" mean in the context of the 510(k) process?
Substantial equivalence means that the new product must be as safe and effective as its predicate, or the already legally marketed product it is compared to.
Which class of devices primarily uses the 510(k) pathway?
The 510(k) pathway primarily applies to Class II devices, which are categorized as moderate-risk.
How long does the FDA 510(k) approval process typically take?
The typical duration for FDA 510(k) approvals is around 33 months, but some applications may take as few as 2 months or extend up to 132 months.
What percentage of 510(k) applications receive a Substantially Equivalent decision?
Approximately 85% of 510(k) applications receive a Substantially Equivalent decision from the FDA.
What is the rejection rate for initial 510(k) submissions?
About 75% of entries are rejected on the first attempt, highlighting the need for thorough preparation.
What is the average cost for companies to achieve FDA 510(k) approvals?
The average cost for companies to achieve FDA 510(k) approvals is approximately $6.1 million.
What is the eSTAR requirement and when did it become mandatory?
The eSTAR requirement is a recent update that became mandatory in October 2023, which manufacturers must consider when preparing their submissions for FDA 510(k) approvals.
What should manufacturers focus on to ensure a successful 510(k) filing?
Manufacturers should focus on comprehensive preparation and a solid understanding of regulatory requirements to ensure a successful filing for FDA 510(k) approvals.