Introduction
Navigating the complexities of clinical trials in Bulgaria demands a comprehensive understanding of the Bulgarian Drug Agency (BDA) approval process. This step is crucial for both researchers and sponsors. This guide provides a clear pathway through the regulatory framework, highlighting the significance of meticulous documentation and timely submissions to facilitate a smooth approval journey. However, challenges such as incomplete applications and potential delays persist.
So, how can one effectively navigate the BDA's stringent requirements and boost the chances of a successful trial initiation?
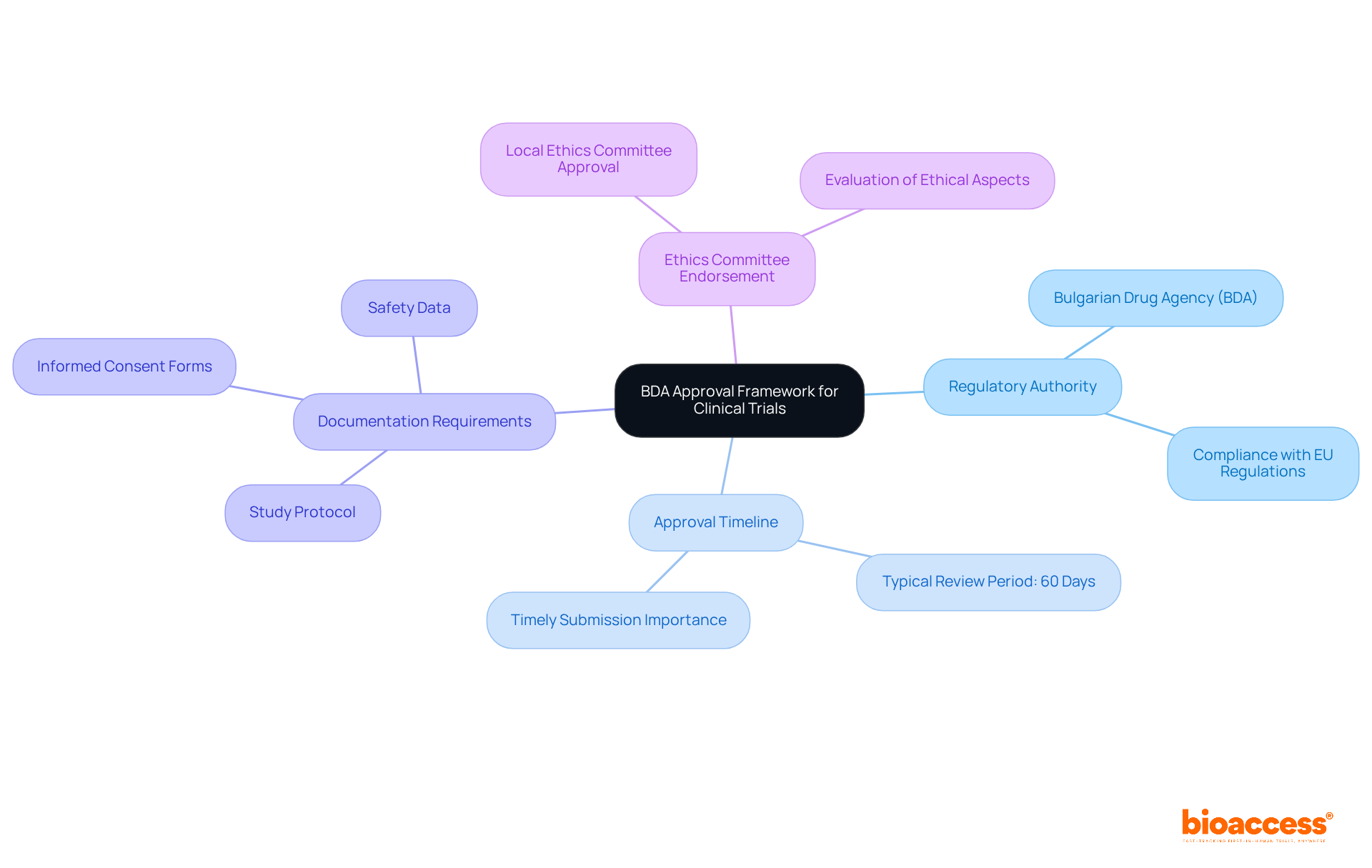
Understand the BDA Approval Framework for Clinical Trials
Navigating the bda approval process for clinical trials in Bulgaria is essential for anyone involved in clinical research. A solid grasp of the Bulgarian Drug Agency (BDA) and its regulatory framework is crucial for success. Operating under the EU Clinical Trials Regulation (EU No 536/2014), the BDA ensures that all clinical studies comply with stringent guidelines.
Key components to familiarize yourself with include:
- Regulatory Authority: The BDA is the main organization responsible for assessing and endorsing clinical trial submissions. Understanding its structure and functions is vital for effectively navigating the approval process.
- Approval Timeline: The BDA typically reviews applications within 60 days. If no objections arise during this period, the proceedings can commence, making timely submission essential.
- Documentation Requirements: A comprehensive set of documents is required by the BDA, including the study protocol, informed consent forms, and safety data. Being aware of these requirements can prevent unnecessary delays in the validation process.
- Ethics Committee Endorsement: In addition to BDA consent, your application must also obtain endorsement from a local ethics committee, which evaluates the ethical aspects of your study.
Understanding these elements is vital for preparing effectively for the subsequent steps in the approval process. With Bulgaria's favorable regulatory environment and efficient review processes, sponsors can benefit from a streamlined pathway to initiate clinical studies.
Gather Required Documentation for Submission
Before submitting your request to the Bulgarian Drug Agency (BDA) as part of the BDA approval process for clinical trials in Bulgaria, it is crucial to compile all necessary documentation meticulously. Here’s a checklist of essential documents to ensure a successful submission:
- Cover Letter: A formal cover letter in Bulgarian, clearly stating the purpose of the submission and listing all involved Member States.
- Form: Complete both Part 1 (general information) and Part 2 (specific details about the experiment) of the form.
- Trial Protocol: A comprehensive protocol detailing the study design, objectives, methodology, and statistical analysis plan.
- Informed Consent Forms: Templates for informed consent that adhere to ethical standards and local regulations, ensuring participants are fully informed before agreeing to join.
- Investigator's Brochure: A thorough document providing essential information about the investigational product, including safety and efficacy data.
- Safety Data: Relevant safety information from prior studies or preclinical trials to support the request.
- Ethics Committee Authorization: Documentation confirming consent from the local ethics committee, which is vital for ethical compliance.
- Fee Payment Document: Ensure to include a fee payment document stating the correct amount and reason for payment, as the BDA will not accept requests without it.
- Archiving Confirmation Documents: Include confirmation documents detailing where and how long essential files will be stored for compliance and future audits.
Ensure that all documents are complete, accurate, and formatted in compliance with the BDA approval process for clinical trials in Bulgaria. This attention to detail will facilitate a smoother submission process and enhance the likelihood of approval. Moreover, keep in mind that the BDA aims for a review period of only 35 days for trial submissions, making prompt and precise documentation essential.

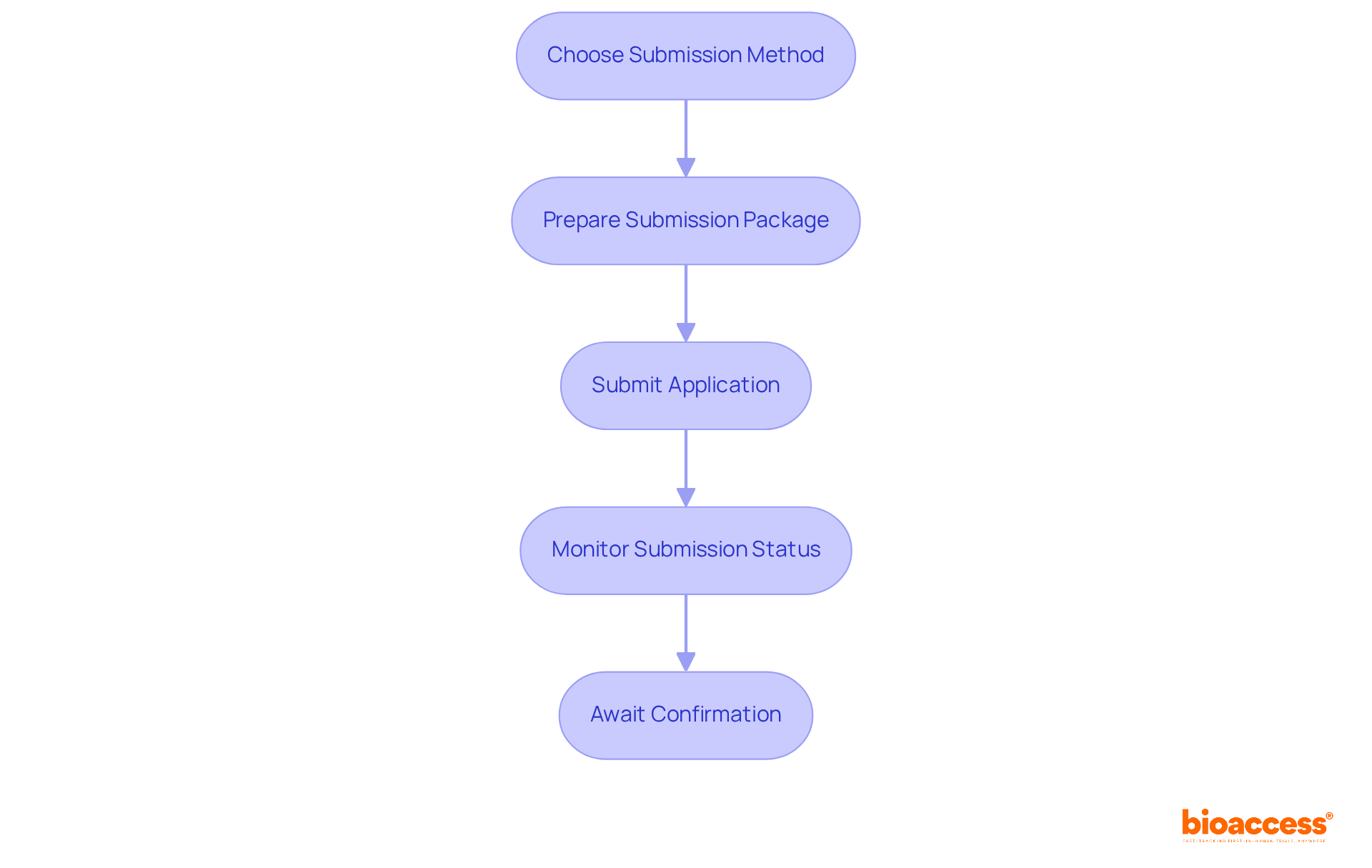
Submit Your Application to the BDA
To successfully submit your application to the Bulgarian Drug Agency (BDA), follow these streamlined steps:
- Choose Submission Method: Decide whether to use the Clinical Trials Information System (CTIS) or submit directly to the BDA. Familiarize yourself with the requirements and the BDA approval process for clinical trials in Bulgaria for both options.
- Prepare Submission Package: Assemble all necessary documents into a comprehensive submission package. Include a cover letter and ensure that each document is clearly labeled to facilitate review.
- Submit Application: For CTIS submissions, adhere to the online process. If choosing direct submission, deliver your package to the BDA’s office in Sofia and obtain a confirmation of receipt to ensure your request is logged.
- Monitor Submission Status: After submission, keep track of your progress. The BDA may reach out for further information or clarification, so be ready to respond promptly.
- Await Confirmation: The BDA intends to evaluate submissions and offer feedback or consent within a 60-day period. Once authorized, you will receive a notification to begin your clinical trial.
By following these steps, you can improve the efficiency and precision of your submission in line with the BDA approval process for clinical trials in Bulgaria.
Troubleshoot Common Issues in the Approval Process
Even with thorough preparation, issues may arise during the BDA approval process for clinical trials in Bulgaria. Understanding these common problems and how to troubleshoot them is essential for success in clinical research.
-
Incomplete Documentation: If the BDA requests additional information, review your submission to identify any missing documents. Ensure all required forms are included and correctly filled out to avoid delays.
-
Delays in Approval: If your request is taking longer than anticipated, contact the BDA for an update. Be prepared to provide any additional information they may require. Notably, the median time from EMA authorization to inclusion in Bulgaria's Positive Drug List (PDL) is approximately 828 days, underscoring the importance of timely communication.
-
Ethics Committee Concerns: If the ethics committee raises concerns, address their feedback promptly. Engage in discussions to clarify any misunderstandings and provide additional data if necessary. The median duration for health technology assessments (HTA) in Bulgaria is around 204 days, indicating the need for efficient resolution of concerns.
-
Language Barriers: Ensure that all documents submitted are in the required languages. If necessary, seek professional translation services to avoid miscommunication and ensure clarity.
-
Regulatory Changes: Stay informed about any changes in regulations that may affect your application. Regularly check the BDA website and relevant EU regulations to ensure compliance.
By being proactive and prepared to address these common issues, you can navigate the BDA approval process for clinical trials in Bulgaria more smoothly, thereby enhancing your chances of success.

Conclusão
Navigating the BDA approval process for clinical trials in Bulgaria is a critical step for researchers aiming to conduct clinical studies successfully. Understanding the Bulgarian Drug Agency's framework, including its regulatory guidelines and documentation requirements, is essential for a smooth approval journey.
This article outlines the key components necessary for navigating this process, such as:
- The importance of timely submission
- The need for comprehensive documentation
- The role of the ethics committee
A well-prepared application can significantly reduce delays and enhance the likelihood of approval, given the BDA's structured review timeline and favorable regulatory environment.
In light of these insights, it is crucial for sponsors and researchers to approach the BDA approval process with diligence and attention to detail. Staying informed about the requirements and potential challenges, and preparing thoroughly, can increase the chances of a successful clinical trial initiation in Bulgaria. Embracing this proactive approach not only facilitates compliance but also contributes to the advancement of clinical research within the region.
Frequently Asked Questions
What is the BDA and its role in clinical trials?
The Bulgarian Drug Agency (BDA) is the main organization responsible for assessing and endorsing clinical trial submissions in Bulgaria, ensuring compliance with the regulatory framework.
What regulations govern clinical trials in Bulgaria?
Clinical trials in Bulgaria operate under the EU Clinical Trials Regulation (EU No 536/2014), which sets stringent guidelines for all clinical studies.
What is the typical approval timeline for clinical trial applications by the BDA?
The BDA typically reviews applications within 60 days. If there are no objections during this period, the clinical trial can commence.
What documentation is required for BDA approval?
A comprehensive set of documents is required, including the study protocol, informed consent forms, and safety data.
Is ethics committee endorsement necessary for clinical trial applications?
Yes, in addition to BDA consent, applications must also obtain endorsement from a local ethics committee that evaluates the ethical aspects of the study.
Why is it important to understand the BDA approval framework?
A solid grasp of the BDA approval framework is crucial for effectively navigating the approval process and preparing for the subsequent steps in clinical trials.
What advantages does Bulgaria offer for clinical trials?
Bulgaria has a favorable regulatory environment and efficient review processes, providing sponsors with a streamlined pathway to initiate clinical studies.
List of Sources
- Understand the BDA Approval Framework for Clinical Trials
- Bulgaria: Rising Hub for Clinical Research | Cromos Pharma (https://cromospharma.com/bulgaria-a-rising-hub-for-clinical-research-in-europe)
- Bulgaria is the sponsor's top choice for conducting clinical trials (https://clinical-trials-bulgaria.com/bulgaria-is-the-sponsors-top-choice-for-conducting-clinical-trials)
- CRO in Bulgaria, Clinical Research in Bulgaria - GCT CRO (https://gctrials.com/coverage/bulgaria)
- Clinical trials in Bulgaria: country profile | Cromos Pharma (https://cromospharma.com/clinical-trials-in-bulgaria-country-profile-for-2023)
- Bulgaria introduces procedure for authorisation of clinical trials per EU regulation (https://cms-lawnow.com/en/ealerts/2022/03/bulgaria-introduces-procedure-for-authorisation-of-clinical-trials-per-eu-regulation)
- Gather Required Documentation for Submission
- Information for companies - Bulgarian Drug Agency (https://bda.bg/en/102-information-for-companies-section)
- 10 Key Insights on eap Regulations for Bulgaria's Clinical Trials (https://bioaccessla.com/blog/10-key-insights-on-eap-regulations-for-bulgarias-clinical-trials)
- ICH-GCP Essential Documents In Clinical Trials Explained : (https://abiogenesisclinpharm.com/essential-documents-in-clinical-trials)
- What Are the Documents Required for Clinical Trial Applications to Regulatory Authorities in Europe? (https://sofpromed.com/what-are-the-documents-required-for-clinical-trial-applications-to-regulatory-authorities-in-europe)
- Submission Process for Medicinal Products – Convex Clinical Research (https://convex.bg/useful-information/submission-process-for-medicinal-products)
- Submit Your Application to the BDA
- Information for companies - Bulgarian Drug Agency (https://bda.bg/en/102-information-for-companies-section)
- 70 Research Quotes to Inspire Your Work - Qualtrics (https://qualtrics.com/articles/strategy-research/research-quotes)
- Bulgaria: Rising Hub for Clinical Research | Cromos Pharma (https://cromospharma.com/bulgaria-a-rising-hub-for-clinical-research-in-europe)
- Submission Process for Medicinal Products – Convex Clinical Research (https://convex.bg/useful-information/submission-process-for-medicinal-products)
- xtalks.com (https://xtalks.com/10-trends-and-statistics-for-clinical-trials-in-2023-3377)
- Troubleshoot Common Issues in the Approval Process
- Bulgaria: Rising Hub for Clinical Research | Cromos Pharma (https://cromospharma.com/bulgaria-a-rising-hub-for-clinical-research-in-europe)
- Access to Orphan Medicinal Products in Bulgaria: An Analysis of the Positive Drug List and Individual Access Schemes - PMC (https://pmc.ncbi.nlm.nih.gov/articles/PMC12469742)
- FDA Delays Push Biotech Companies to Rethink U.S. Drug Development Strategy (https://veristat.com/blog/fda-delays-push-biotech-companies-to-rethink-u.s.-drug-development-strategy)
- FDA to lower number of trials required for approval of drugs, other medical products (https://statnews.com/2025/12/04/fda-considers-single-clinical-trial-for-new-product-approvals)
- 23 Must-Read Quotes About Data [& What They Really Mean] (https://careerfoundry.com/en/blog/data-analytics/inspirational-data-quotes)






