


The integrity of clinical trials relies heavily on the vigilant oversight of ethics committees, often seen as the guardians of participant welfare. These committees hold a crucial responsibility: ensuring that research adheres to ethical standards while prioritizing the rights and safety of individuals involved in studies. Yet, the effectiveness of these committees faces challenges, particularly due to gaps in statistical expertise and differing interpretations of ethical requirements.
How can ethics committees navigate these complexities? They must uphold their vital role in advancing medical research while safeguarding participant trust. This balance is essential not only for the integrity of clinical trials but also for fostering public confidence in the research process.
The pivotal role in clinical trials is played by ethics committees, also known as Institutional Review Boards (IRBs), which are responsible for ethics committee responsibilities in clinical trials. The ethics committee responsibilities in clinical trials primarily focus on protecting the rights, safety, and welfare of trial participants. The ethics committee responsibilities in clinical trials include:
However, the effectiveness of these committees can be significantly influenced by the availability of statistical expertise. Alarmingly, only 25% of committees had a qualified statistician when qualifications were considered, raising concerns about the adequacy of statistical oversight. While 60% of human ethics committees in Australia have access to a qualified statistician, there remains considerable variability in attitudes towards the necessity of statisticians in these committees. Some members assert that statistical input is essential, while others believe it is only necessary for intricate analyses.
Furthermore, the moral consequences of study waste resulting from insufficient statistical examination cannot be overlooked. The ethics committee responsibilities in clinical trials play a crucial role in reducing study waste and safeguarding participant safety by ensuring that investigations are designed and executed with proper statistical oversight. Ultimately, the ethics committee responsibilities in clinical trials serve as guardians of ethical conduct in clinical research, reinforcing the commitment to participant welfare and the advancement of medical knowledge.

Ethics committees play a pivotal role in clinical trials, ensuring the integrity and ethical conduct of research through several key responsibilities:
Reviewing Research Proposals: Ethics committees meticulously assess the scientific validity and moral implications of proposed projects. This procedure is essential for protecting participant well-being and ensuring that project goals align with moral standards. Their role is further enhanced by Bioaccess's extensive clinical trial management services, including feasibility assessments and site selection, which guarantee that the appropriate conditions are established for ethical research.
Informed Consent Oversight: Committees are responsible for ensuring that participants receive comprehensive information about the study, including potential risks and benefits. This oversight is vital; studies indicate that informed consent reporting is highest in clinical trials at 94.6%, yet only 21.3% of studies overall reported informed consent. This stark contrast highlights the need for rigorous adherence to this ethical requirement and underscores the importance of transparency in ethical disclosures, particularly in sensitive areas like mental health and AI technologies.
Monitoring Current Research: Regular evaluations of ongoing investigations are conducted by ethics boards to ensure adherence to moral standards and participant safety. This monitoring is essential, as it helps identify any deviations from approved protocols and addresses them promptly. Bioaccess supports this process through effective project management and monitoring, ensuring that all aspects of the trial adhere to ethical guidelines.
Addressing Participant Concerns: Ethics committees serve as a platform for participants to voice concerns or complaints regarding the study. This responsibility promotes trust and transparency, enabling participants to feel acknowledged and appreciated throughout the study process.
Ensuring Adherence to Regulations: Committees guarantee that all investigative activities conform to local and international moral guidelines and regulations. This includes adherence to frameworks like GDPR and HIPAA, which are critical for protecting participant rights and data privacy. Furthermore, the global variation in how moral standards are implemented and reported presents challenges that ethics groups must address. This highlights the necessity for interdisciplinary collaboration among ethicists, scholars, and journal editors to enhance moral standards in studies. Bioaccess's expertise in trial setup, compliance reviews, and site selection further enhances the ability of ethics committees to uphold these standards.
In summary, the ethics committee responsibilities in clinical trials are essential for upholding moral standards in clinical research, ensuring that participant safety and rights are prioritized throughout the research process.

The ethics committee review process stands as a cornerstone in ensuring the ethical conduct of clinical trials, encompassing several key stages:
Submission of Documents: Researchers initiate the process by submitting their research protocol, informed consent forms, and any additional necessary documentation to the ethics board. This initial submission is vital for laying the groundwork for the review.
Initial Review: The committee performs an initial review to determine whether the submission is complete and meets fundamental ethical standards. This step is crucial for identifying any immediate concerns that could impede the review process.
Full Committee Review: Should the study be assessed as posing more than minimal risk, it proceeds to a full committee review. During this phase, committee members engage in thorough discussions regarding the proposal, evaluating its ethical implications and scientific validity.
Feedback and Revisions: Following the comprehensive evaluation, the committee provides feedback to the researchers. This feedback often necessitates modifications to the research protocol or informed consent documents, ensuring that all ethical concerns are adequately addressed before moving forward.
Approval or Rejection: Once all issues have been resolved, the committee either approves the study or outlines the reasons for rejection. Researchers can then address these reasons in resubmissions, fostering a collaborative approach to ethical compliance.
Data indicates that approximately 45% of applications receive approval during the initial review, while 7% face rejection. This statistic underscores the importance of meticulous preparation and adherence to standards in the submission process. Moreover, the likelihood of securing a favorable opinion can be significantly influenced by the quality of informed consent and the principal investigator's leadership role, with odds ratios revealing a strong correlation between these factors and successful outcomes. As highlighted by Luis Vivanco, in Spain, biomedical research applications must obtain a positive ethical opinion from Research Ethics Committees (RECs) prior to execution, which emphasizes the ethics committee responsibilities in clinical trials during the review process. Additionally, the pandemic has tested the capacity of RECs to uphold ethical standards amid a surge in submissions, further complicating the review landscape.

Ethics committees face a variety of challenges and ethical dilemmas in their oversight of clinical trials, including:

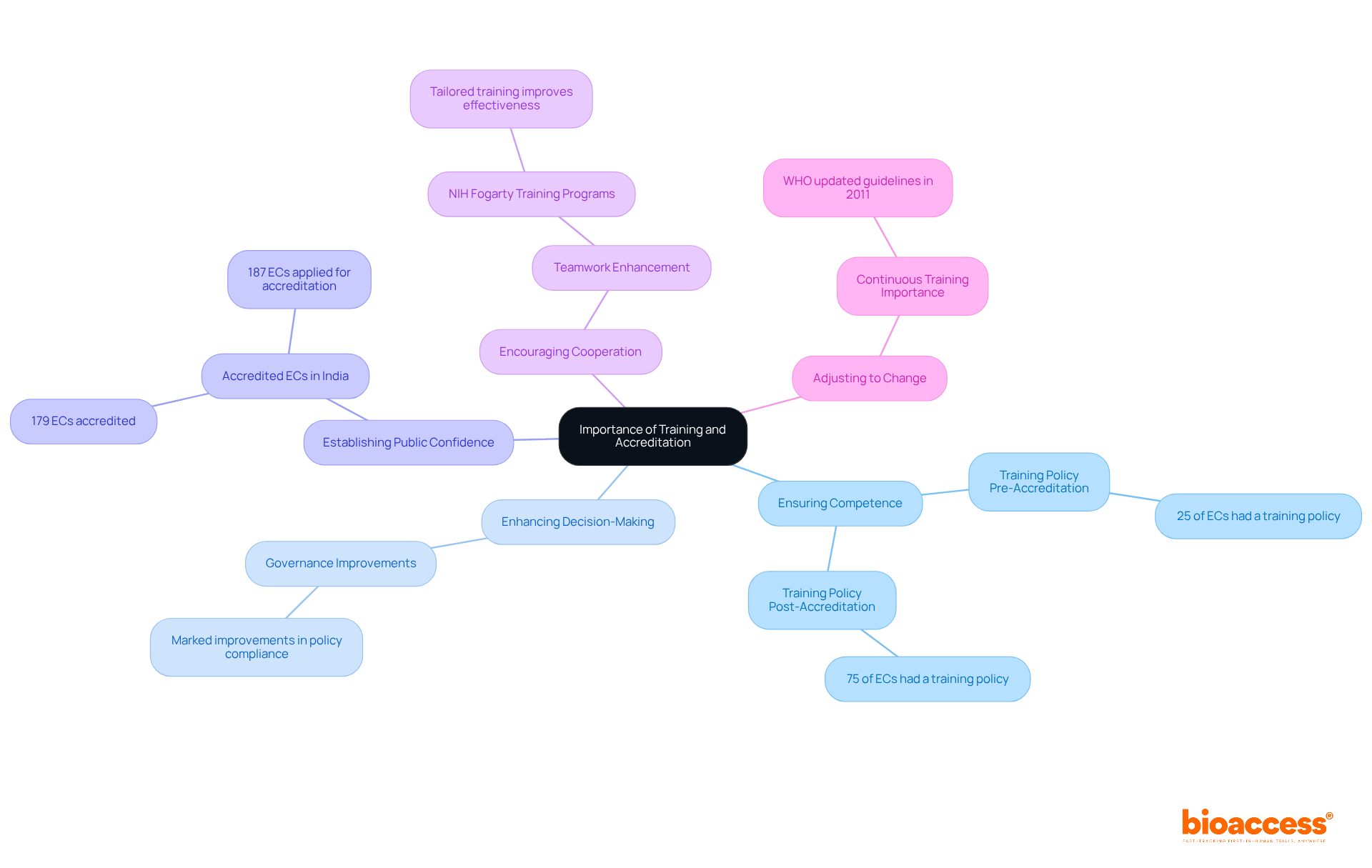
Training and accreditation for ethics committee members are vital in the realm of clinical research for several compelling reasons:
Ensuring Competence: Ongoing education is essential for committee members to stay abreast of the latest ethical guidelines, regulations, and best practices in clinical research. Notably, an analysis revealed that 25% of ethics committees (ECs) had a training policy for members prior to accreditation. This figure surged to 75% post-accreditation, significantly enhancing their operational standards.
Enhancing Decision-Making: Well-trained members are far better equipped to make informed decisions regarding study approvals and participant safety. Research indicates that the ethics committee responsibilities in clinical trials of accredited ECs show marked improvements in governance and adherence to ethical standards, with significant differences in policy compliance between accredited and non-accredited committees.
Establishing Public Confidence: Accreditation signifies a commitment to high moral standards, fostering public trust in the research process. With 179 ECs accredited in India and 187 having applied for accreditation from NABH, their recognition of adherence to established guidelines bolsters the credibility of clinical trials conducted in the region.
Encouraging Cooperation: Training programs often enhance teamwork among committee members, improving their ability to tackle complex ethical dilemmas. For example, the NIH Fogarty International Training Programs have demonstrated that tailored training can significantly enhance the capacity and effectiveness of research ethics committees in low-resource settings.
Adjusting to change: Continuous training enables ethics committees to effectively manage their ethics committee responsibilities in clinical trials by confronting emerging ethical challenges and regulatory requirements. The World Health Organization's updated guidelines in 2011 underscore the necessity for comprehensive standards, highlighting the critical role of ongoing education in maintaining the quality of ethical oversight in clinical research.
Ethics committees, or Institutional Review Boards (IRBs), serve as essential guardians in the realm of clinical trials, tasked with the critical responsibility of ensuring that research is conducted ethically while prioritizing the rights and welfare of participants. Their multifaceted roles include:
The effectiveness of these committees is significantly enhanced by the inclusion of qualified statisticians, a factor that greatly influences the overall integrity of clinical research.
Throughout this discussion, several key responsibilities of ethics committees have been highlighted, including their functions in:
The challenges faced by these committees, such as:
have also been illuminated. Furthermore, the importance of continuous training and accreditation for committee members emerges as a vital aspect of enhancing their capabilities and fostering public trust in the clinical research process.
Given the complexities and ethical dilemmas inherent in clinical trials, the role of ethics committees cannot be overstated. Their unwavering commitment to upholding ethical standards is crucial not only for safeguarding participant welfare but also for advancing the integrity of medical research. As the landscape of clinical trials continues to evolve, fostering a culture of continuous education and collaboration within ethics committees will be essential in addressing emerging challenges and ensuring that ethical oversight remains robust and effective. Engaging in these efforts is imperative for the future of ethical clinical research, ultimately benefiting both participants and the broader medical community.
What is the role of ethics committees in clinical trials?
Ethics committees, also known as Institutional Review Boards (IRBs), are responsible for protecting the rights, safety, and welfare of trial participants. They ensure that research adheres to ethical standards and regulatory requirements, maintain the integrity of clinical trials, and foster public trust in medical research.
What are the key responsibilities of ethics committees in clinical trials?
Key responsibilities include reviewing research proposals for scientific validity and moral implications, overseeing informed consent processes, monitoring ongoing research for adherence to ethical standards, addressing participant concerns, and ensuring compliance with local and international regulations.
How do ethics committees ensure informed consent is properly managed?
Ethics committees ensure that participants receive comprehensive information about the study, including potential risks and benefits. They oversee the informed consent process to ensure transparency and adherence to ethical requirements, which is crucial for participant well-being.
Why is statistical expertise important for ethics committees?
Statistical expertise is vital for ethics committees as it helps ensure proper statistical oversight in clinical trials. Only 25% of committees had a qualified statistician, raising concerns about the adequacy of statistical examination and the potential for study waste due to insufficient statistical input.
How do ethics committees address participant concerns during clinical trials?
Ethics committees provide a platform for participants to voice concerns or complaints regarding the study, promoting trust and transparency and ensuring that participants feel acknowledged throughout the research process.
What regulations do ethics committees ensure compliance with?
Ethics committees ensure that all investigative activities conform to local and international moral guidelines and regulations, including frameworks like GDPR and HIPAA, which protect participant rights and data privacy.
What is the significance of monitoring ongoing research by ethics committees?
Regular evaluations of ongoing investigations by ethics committees are essential to ensure adherence to moral standards and participant safety. This monitoring helps identify any deviations from approved protocols and allows for prompt corrective actions.