


Achieving UDI audit readiness in Mexico necessitates a comprehensive understanding of UDI requirements. It is imperative to:
This article outlines these critical steps, emphasizing the importance of:
Such measures are essential to ensure compliance with evolving standards and to navigate the complexities of the Medtech landscape effectively.
Understanding the intricacies of Unique Device Identification (UDI) requirements is essential for medical device manufacturers in Mexico, particularly given the evolving regulatory landscape poised to impact compliance by 2025. This article delineates the critical steps necessary for achieving UDI audit readiness, from comprehending the foundational guidelines to implementing effective training and continuous improvement strategies. As organizations navigate these complex requirements, a pressing question emerges: how can they ensure not only compliance but also adopt a proactive stance in response to regulatory changes?
To achieve UDI audit readiness Mexico, it is crucial to understand the UDI guidelines set by COFEPRIS, the Mexican regulatory authority. Key aspects to consider include:
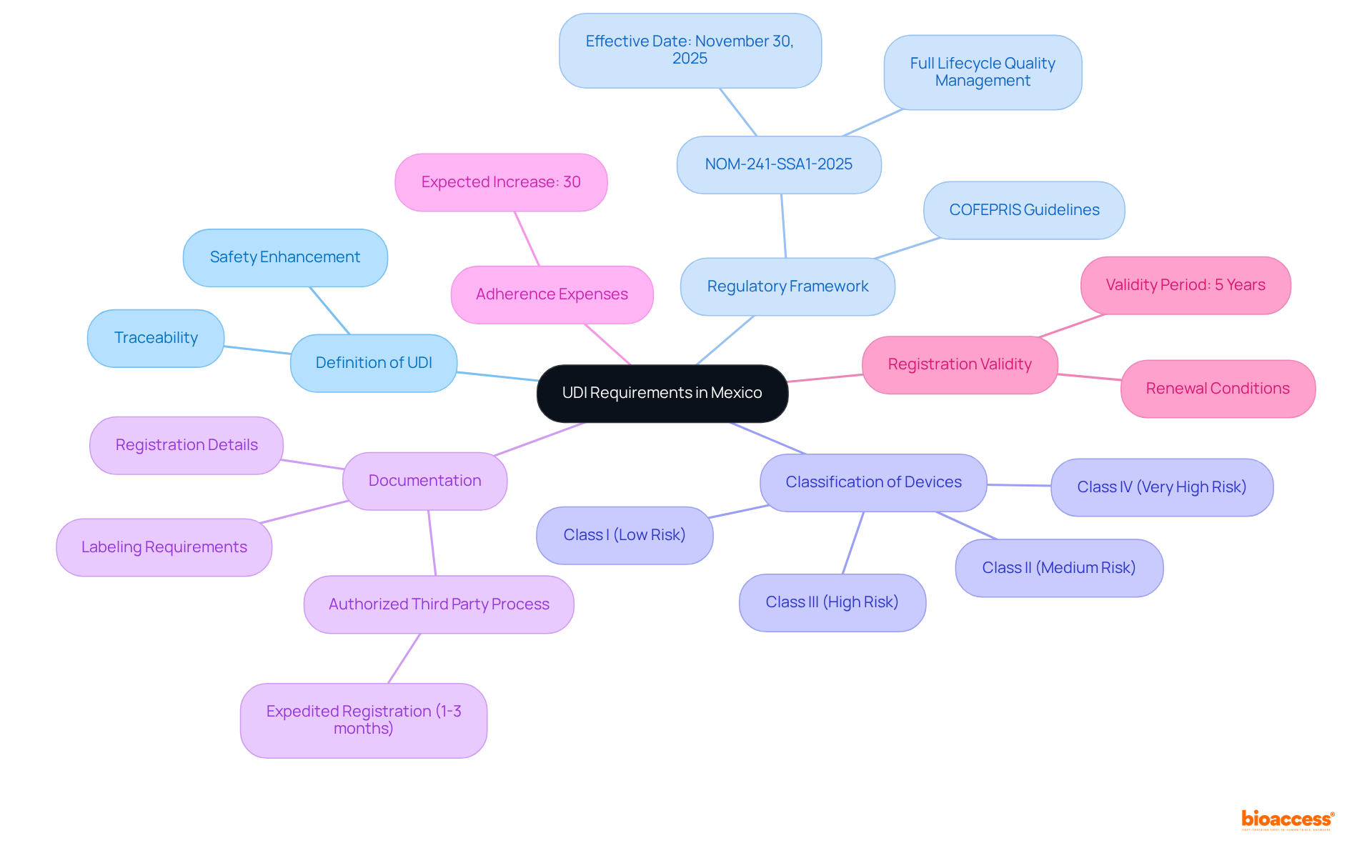
Definition of UDI: UDI, or Unique Device Identifier, is a critical identifier assigned to medical devices that enhances traceability and safety throughout the supply chain.
Regulatory Framework: Stay informed about the latest regulations, especially NOM-241-SSA1-2025, which outlines UDI specifications and adherence timelines. This standard will take effect on November 30, 2025, and emphasizes full lifecycle quality and risk management. Notably, all references to UDI have been removed from this standard, confirming COFEPRIS’s pause on the UDI system.
Classification of Devices: Understanding how your device is classified under Mexican regulations is crucial, as this classification directly influences the specific UDI requirements that apply. COFEPRIS classifies medical devices into four main groups: Class I (low risk), Class II (medium risk), Class III (high risk), and Class IV (very high risk).
Documentation: Compile the necessary documentation for UDI adherence, which includes labeling, registration details, and any additional information mandated by COFEPRIS. The registration process can be expedited through an Authorized Third Party, potentially reducing the timeline to as fast as 1 to 3 months.
Adherence Expenses: Be aware that adherence expenses are expected to increase by an average of 30% due to the new NOM-241-SSA1-2025 standard.
Registration Validity: The validity of registration with COFEPRIS is 5 years and can be renewed if no substantial changes occur.
By mastering these elements, you will be well-prepared for UDI audit readiness Mexico and ensure adherence to the evolving regulatory environment.
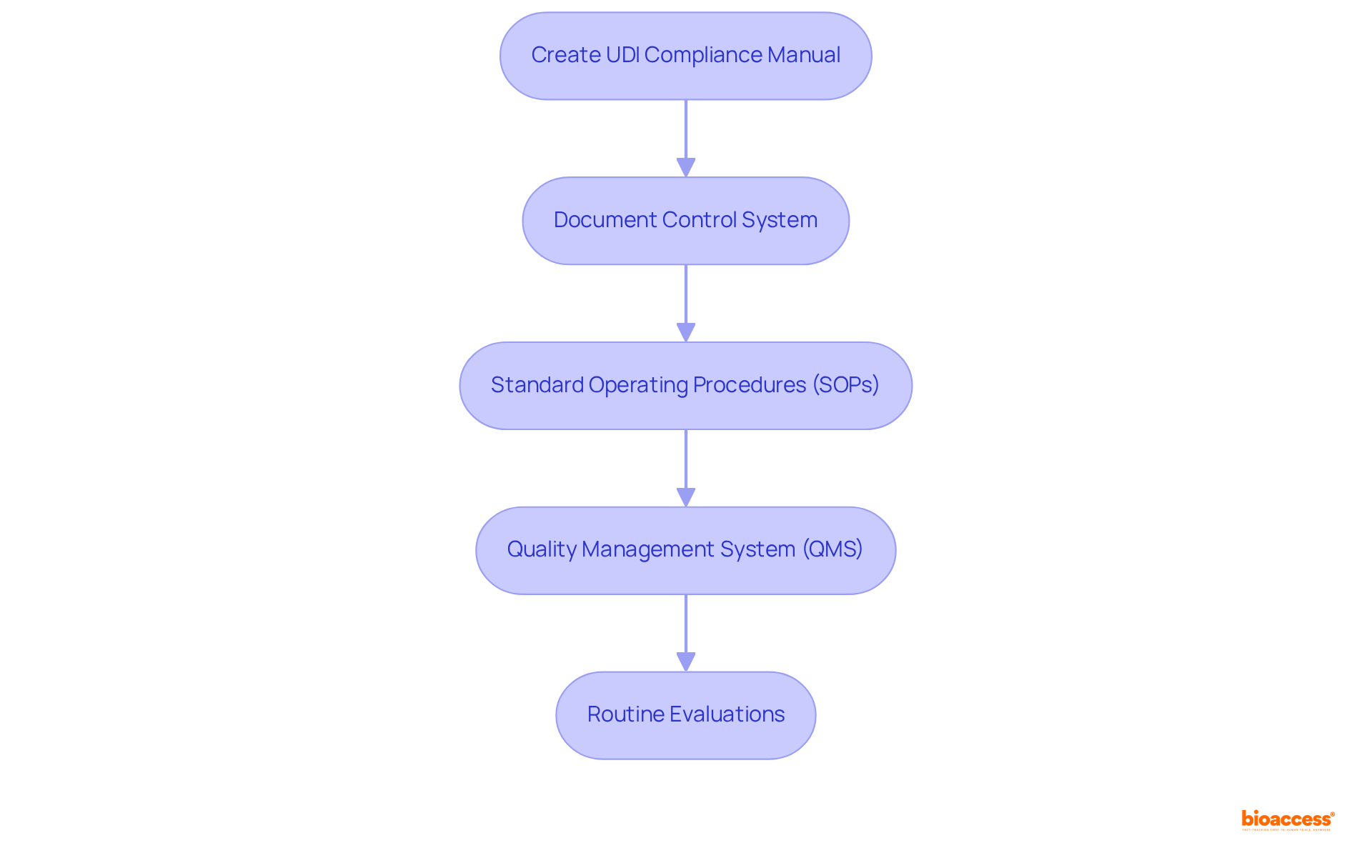
To establish effective documentation and compliance processes, it is essential to follow these steps:
By implementing these procedures, you will establish a solid foundation for UDI compliance.
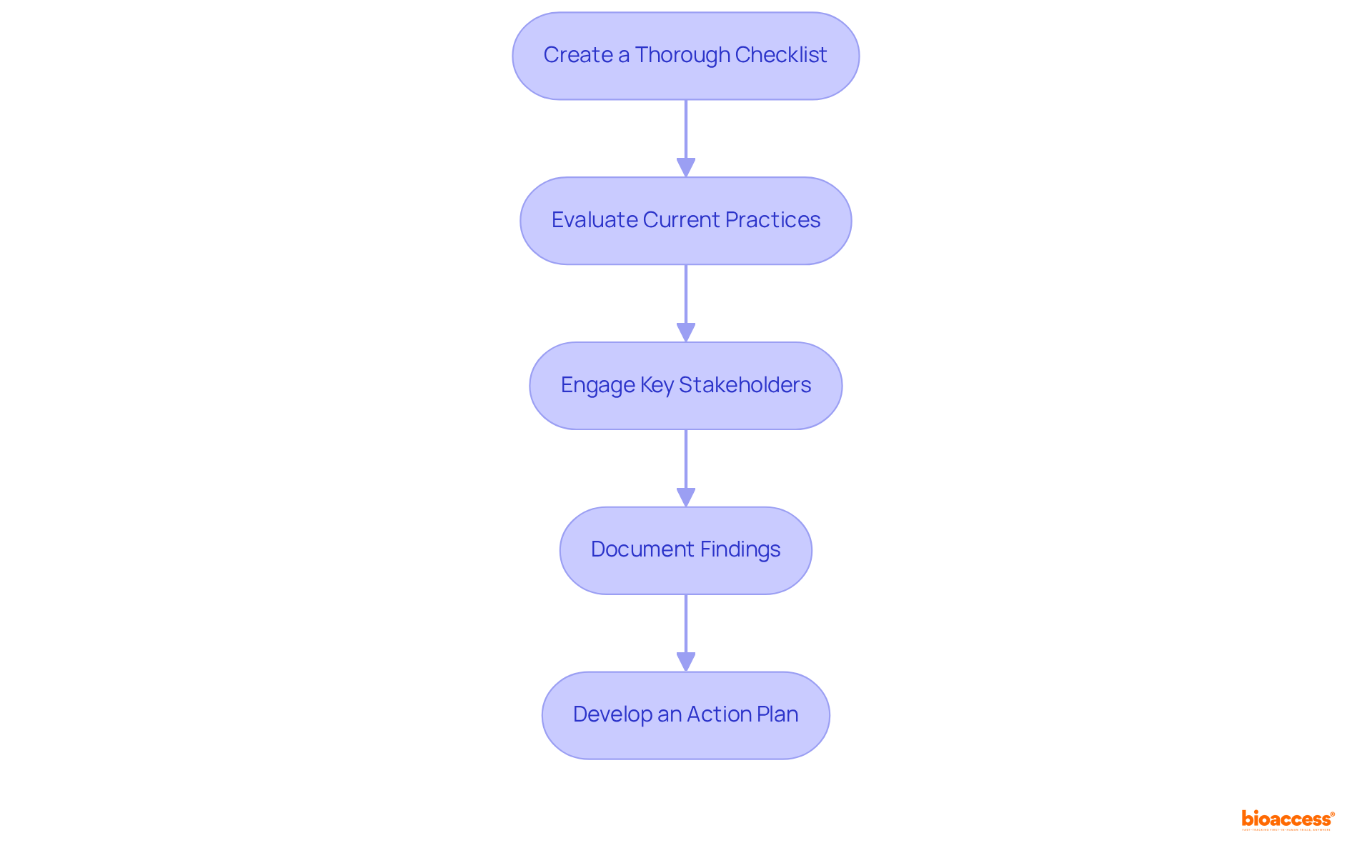
To conduct a self-assessment for UDI readiness, follow these essential steps:
By conducting this self-evaluation, you will gain a comprehensive understanding of your UDI audit readiness in Mexico and identify the specific areas that require enhancement, ultimately improving your regulatory stance.
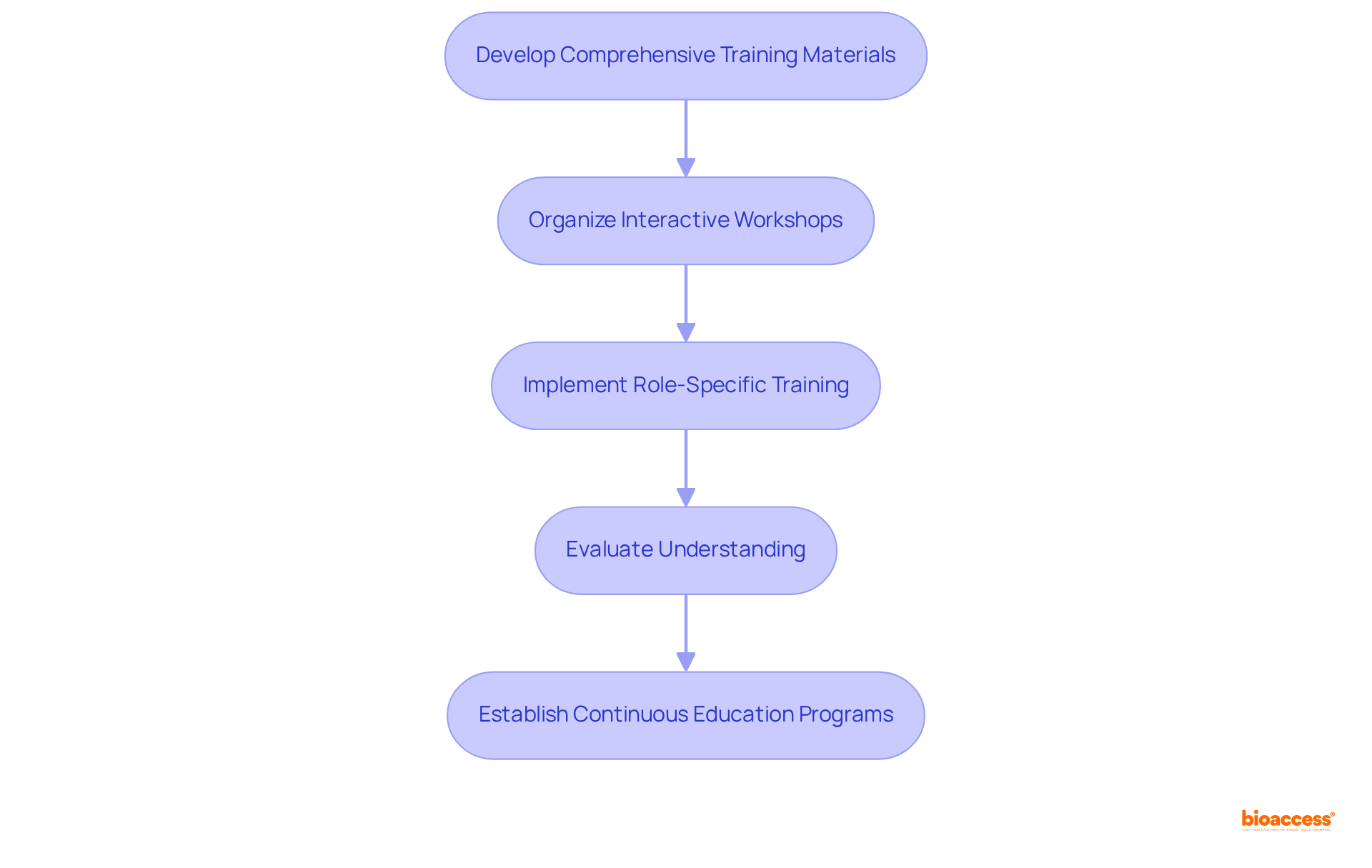
To effectively prepare your team for UDI compliance and audit procedures, it is crucial to follow these essential steps:
Develop Comprehensive Training Materials: Create detailed training resources that encompass UDI requirements, adherence processes, and audit expectations. Ensure these materials are accessible and easy to understand. UDI comprises two primary elements: the Device Identifier (DI) and the Production Identifier (PI), which are essential for adherence.
Organize Interactive Workshops: Facilitate workshops and training sessions that encourage participation and interactive learning. Engaging formats can enhance retention and understanding of UDI concepts.
Implement Role-Specific Training: Customize training sessions to address the specific responsibilities of different roles within your organization. This guarantees that each team member understands their distinct contributions to UDI regulations.
Evaluate Understanding: Use assessments or quizzes to gauge team members' grasp of UDI requirements and procedures. Regular evaluations help identify knowledge gaps and reinforce learning. Conducting regular data audits is essential for UDI audit readiness in Mexico to ensure data quality, as emphasized by the FDA: 'Data quality is critical to the success of the UDI system.' Manufacturers must ensure that their UDI data is accurate, complete, and consistent to avoid regulatory issues and ensure patient safety.
Establish Continuous Education Programs: Develop ongoing education initiatives to keep your team informed about regulatory updates and best practices. This proactive method promotes a culture of adherence and adaptability. Achieving UDI conformity can be costly due to expenses related to labeling technology, data management, and training, so it's important to plan accordingly.
Investing in comprehensive training not only improves your organization's adherence capabilities but also equips your team to handle the intricacies of UDI regulations effectively.
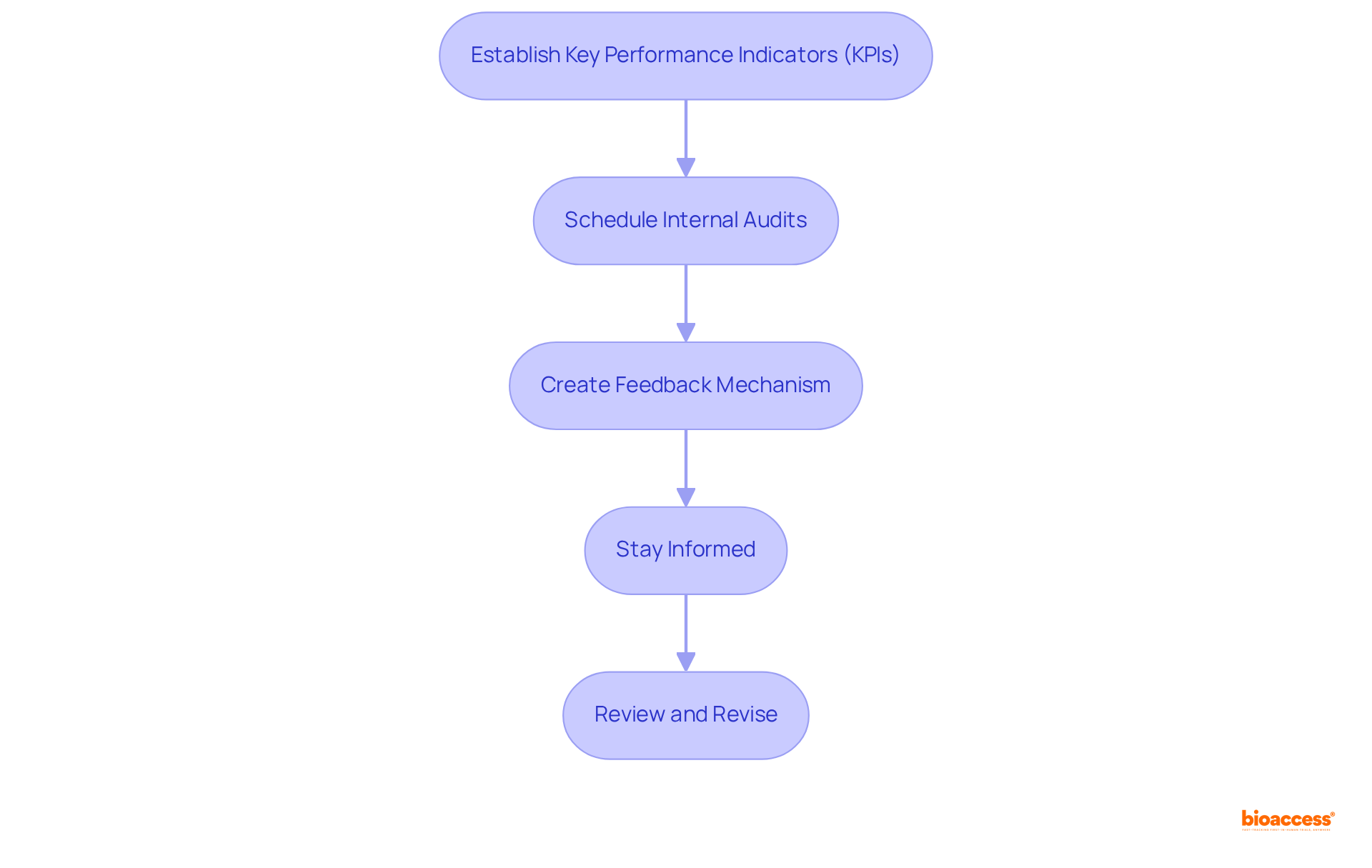
To effectively implement continuous monitoring and improvement strategies for UDI compliance, consider the following steps:
By implementing these strategies, you will foster a culture of continuous improvement, ensuring sustained UDI compliance and enhancing operational efficiency while mitigating the risks associated with poor compliance management.
Achieving UDI audit readiness in Mexico necessitates a comprehensive understanding of the unique device identifier (UDI) requirements established by COFEPRIS, the regulatory authority. By thoroughly familiarizing oneself with the UDI guidelines—encompassing the classification of medical devices and the requisite documentation—organizations can lay a solid foundation for compliance. The forthcoming NOM-241-SSA1-2025 standard underscores the critical importance of adhering to UDI regulations while preparing for the associated costs and processes.
Key steps to ensure UDI readiness encompass:
Each of these components is vital in identifying gaps, enhancing knowledge, and fostering a culture of compliance within organizations. By actively engaging stakeholders and routinely updating practices, businesses can adeptly navigate the evolving regulatory landscape.
Ultimately, the journey toward UDI audit readiness transcends mere regulatory compliance; it is fundamentally about ensuring patient safety and maintaining operational efficiency. Organizations are strongly encouraged to prioritize UDI compliance as a strategic initiative, investing in training and continuous improvement to adeptly navigate the complexities of the medical device industry. Embracing these practices not only enhances compliance but also significantly contributes to the overall success and sustainability of medical device operations in Mexico.
What is UDI and why is it important?
UDI, or Unique Device Identifier, is a critical identifier assigned to medical devices that enhances traceability and safety throughout the supply chain.
What are the key regulations regarding UDI in Mexico?
The key regulation is NOM-241-SSA1-2025, which outlines UDI specifications and adherence timelines. This standard will take effect on November 30, 2025, and emphasizes full lifecycle quality and risk management.
How does COFEPRIS classify medical devices?
COFEPRIS classifies medical devices into four groups based on risk: Class I (low risk), Class II (medium risk), Class III (high risk), and Class IV (very high risk).
What documentation is required for UDI adherence in Mexico?
Necessary documentation includes labeling, registration details, and any additional information mandated by COFEPRIS.
How can the registration process for UDI adherence be expedited?
The registration process can be expedited through an Authorized Third Party, potentially reducing the timeline to as fast as 1 to 3 months.
What should companies expect in terms of adherence expenses due to the new UDI regulations?
Adherence expenses are expected to increase by an average of 30% due to the new NOM-241-SSA1-2025 standard.
What is the validity period for registration with COFEPRIS?
The validity of registration with COFEPRIS is 5 years and can be renewed if no substantial changes occur.
What steps should be taken to establish effective documentation and compliance processes for UDI?
Steps include creating a UDI Compliance Manual, implementing a document control system, establishing Standard Operating Procedures (SOPs), integrating UDI into the Quality Management System (QMS), and scheduling regular evaluations of documentation and procedures.