


The article delineates five essential steps for planning successful pivotal studies in clinical research. These steps encompass:
Each step is underpinned by detailed strategies and best practices, underscoring the significance of robust planning and execution. This meticulous approach is crucial to meet regulatory standards and augment the likelihood of successful outcomes in clinical trials.
Understanding the intricacies of pivotal studies is essential in the realm of clinical research, where the stakes are high and the outcomes can shape the future of medical treatments. These Phase III evaluations not only validate the effectiveness and safety of new therapies but also play a critical role in securing regulatory approvals. However, the path to successful pivotal studies is fraught with challenges, including:
How can researchers ensure that their pivotal studies yield meaningful results and meet the stringent demands of regulatory bodies? This guide outlines five essential steps to effectively plan and execute pivotal studies, unlocking the potential for groundbreaking advancements in healthcare.
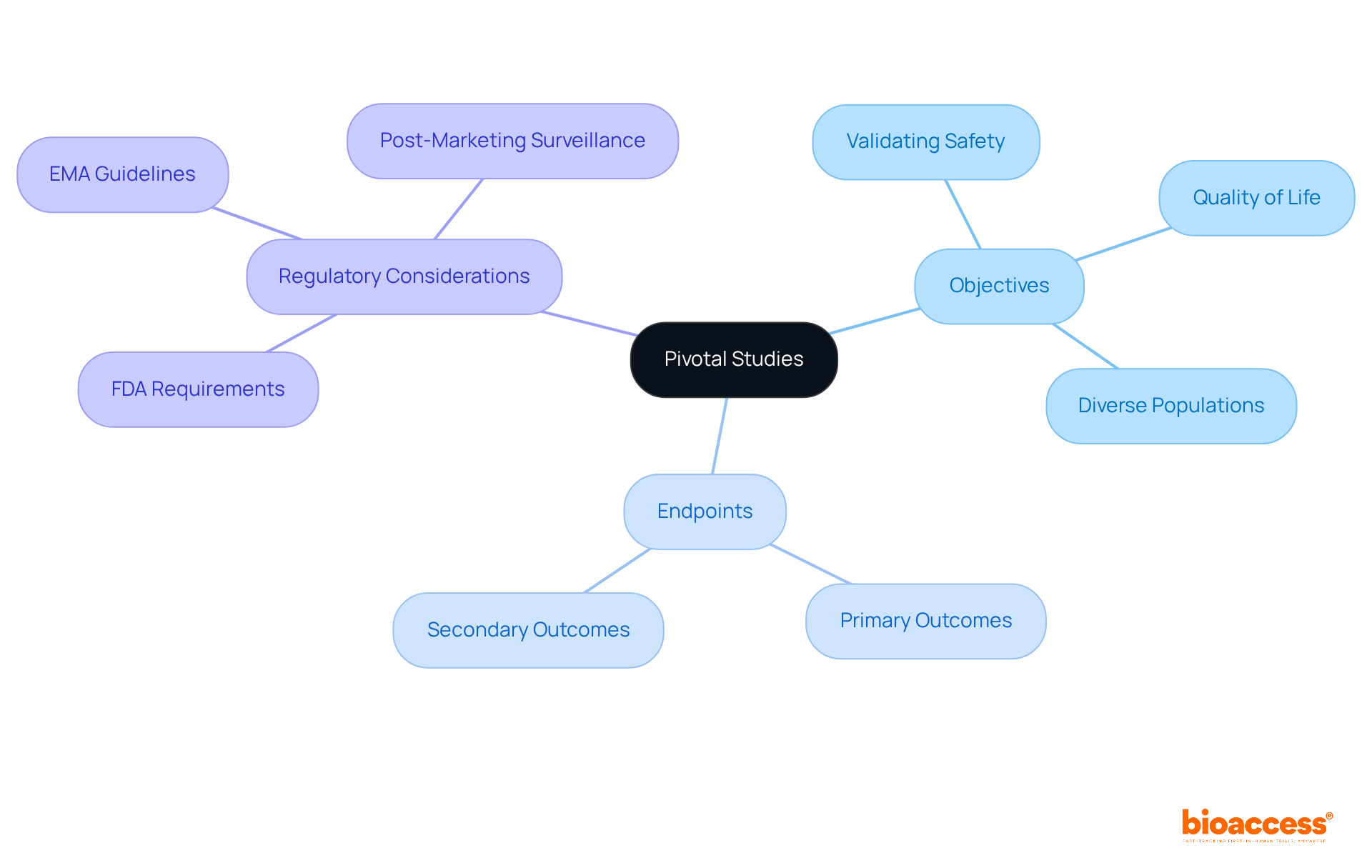
Pivotal studies, often referred to as Phase III clinical evaluations, are essential for determining the effectiveness and safety of new medications or medical devices. These studies typically involve a substantial participant pool, ranging from 300 to 3,000 individuals, which is essential for generating robust data required for regulatory approval from organizations such as the FDA and EMA. The primary objectives of Phase III studies include:
Endpoints in these investigations often encompass both primary outcomes, such as the drug's effectiveness, and secondary outcomes, which may include safety profiles and additional benefits. Understanding the regulatory landscape and the specific data requirements for pivotal studies is crucial, as it directly influences the planning and execution of successful research.
With the increasing complexity of medical trials, staying informed on evolving regulatory standards and integrating adaptive trial designs can significantly enhance the likelihood of achieving favorable results and securing essential approvals. By leveraging bioaccess®'s expertise in critical research areas, including services like regulatory approval and patient recruitment, along with a focus on regions such as Latin America, Eastern Europe, and Australia, you can elevate your chances of success in clinical research.
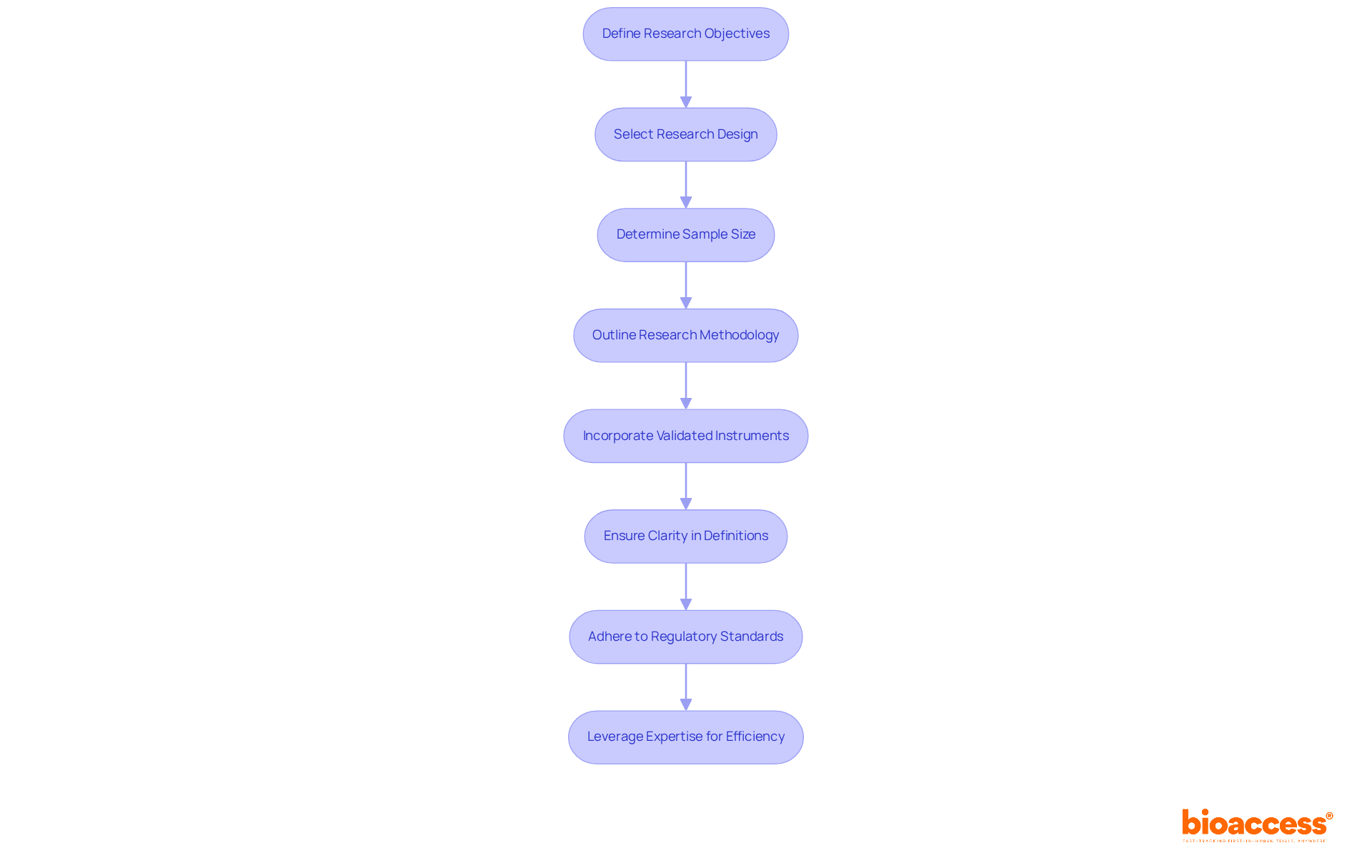
To commence a successful and crucial investigation, it is essential to clearly define your research objectives and the specific questions you aim to address, as these pivotal studies will guide your research design. Selecting a suitable research design—such as pivotal studies or randomized controlled experiments—that aligns with your objectives is imperative. Pivotal studies, such as randomized controlled studies, are frequently favored for their capacity to reduce bias and determine causality.
Determining the sample size is crucial for achieving statistical significance. Consider factors such as expected effect size and variability; for instance, phase III clinical trials typically involve several hundred patients to ensure robust data. A well-organized protocol should outline the research methodology, including participant selection criteria, data collection methods, and statistical analysis plans, while also detailing pivotal studies, timelines, and resource allocation for smooth execution.
Incorporating validated instruments and measures enhances the reliability of your findings. Clarity in operational definitions of disease states and health outcomes is essential, as highlighted in good research practice guidelines. By adhering to these principles, you can create a crucial research project that meets regulatory standards and provides valuable insights to the field. Additionally, leveraging bioaccess®'s expertise can facilitate accelerated patient enrollment, achieving cohorts 50% faster than traditional methods and potentially saving $25K per patient, thus enhancing the overall efficiency of your clinical trial.
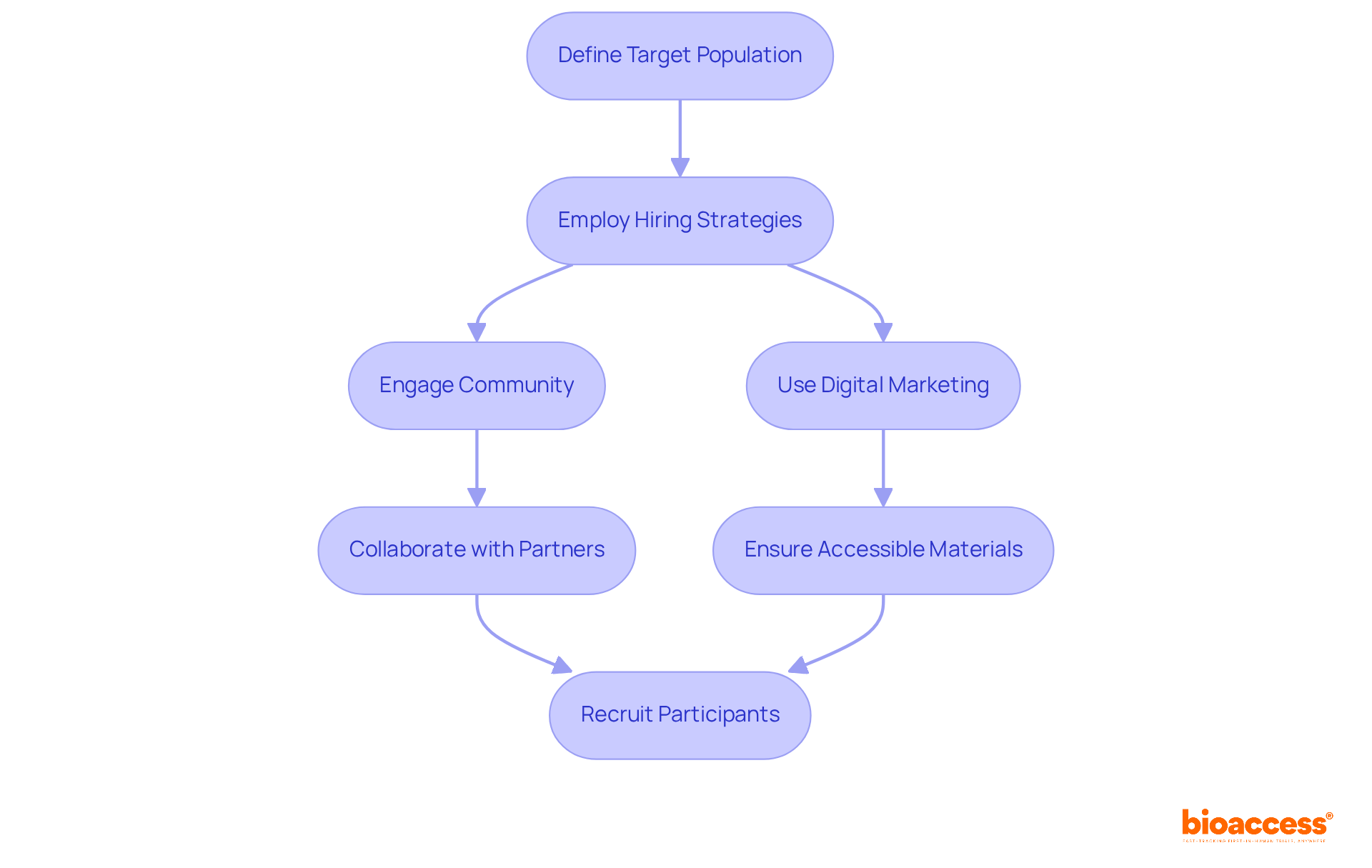
To successfully recruit qualified participants, it is essential to begin by clearly defining your target population using the study's inclusion and exclusion criteria. This foundational step guarantees that hiring efforts are focused and effective. Employ a diverse range of hiring strategies, including:
Notably, community involvement can significantly enhance recruitment success; research indicates that support from the community can elevate patient satisfaction to levels comparable to family support. Furthermore, the majority of the general public prefers to hear about a study from their primary care provider, establishing this as a critical channel for outreach.
In the context of Barranquilla, Colombia, the partnership between bioaccess™ and Caribbean Health Group aims to position the city as a leading site for research trials in Latin America, with the backing of Colombia's Minister of Health. This initiative underscores the importance of local collaborations in refining hiring strategies and fostering a robust research environment. Importantly, alliances such as that of GlobalCare Clinical Trials with bioaccess™ have demonstrated the capacity to achieve over a 50% reduction in participant sourcing duration and 95% retention rates, emphasizing the effectiveness of strategic partnerships in research.
Ensure that your recruitment materials are accessible and informative, ideally tailored to a fourth to sixth-grade reading level, to effectively engage potential participants. This approach is vital, as clear communication can bridge gaps in understanding and encourage participation. Additionally, assembling a team of skilled researchers with substantial experience in conducting medical studies is crucial. Their expertise will not only guide participants through the research process but also ensure compliance with protocols, ultimately enhancing the integrity and success rates of the study.
Integrating various hiring strategies is indispensable, as data reveals that over 90 percent of research studies fail to attract the necessary number of participants. By utilizing a combination of traditional and online outreach, including social media, you can connect with a broader audience and improve hiring outcomes. Engaging with marginalized groups is particularly important; surveys show that:
express a willingness to join trials, highlighting the need for inclusive strategies to attract participants. Moreover, facilitating patients' initial site visits can increase randomization rates by threefold, further enhancing enrollment efforts.
Ultimately, a multifaceted strategy that prioritizes community involvement and transparent communication will enhance participant recruitment and retention, paving the way for successful research outcomes.
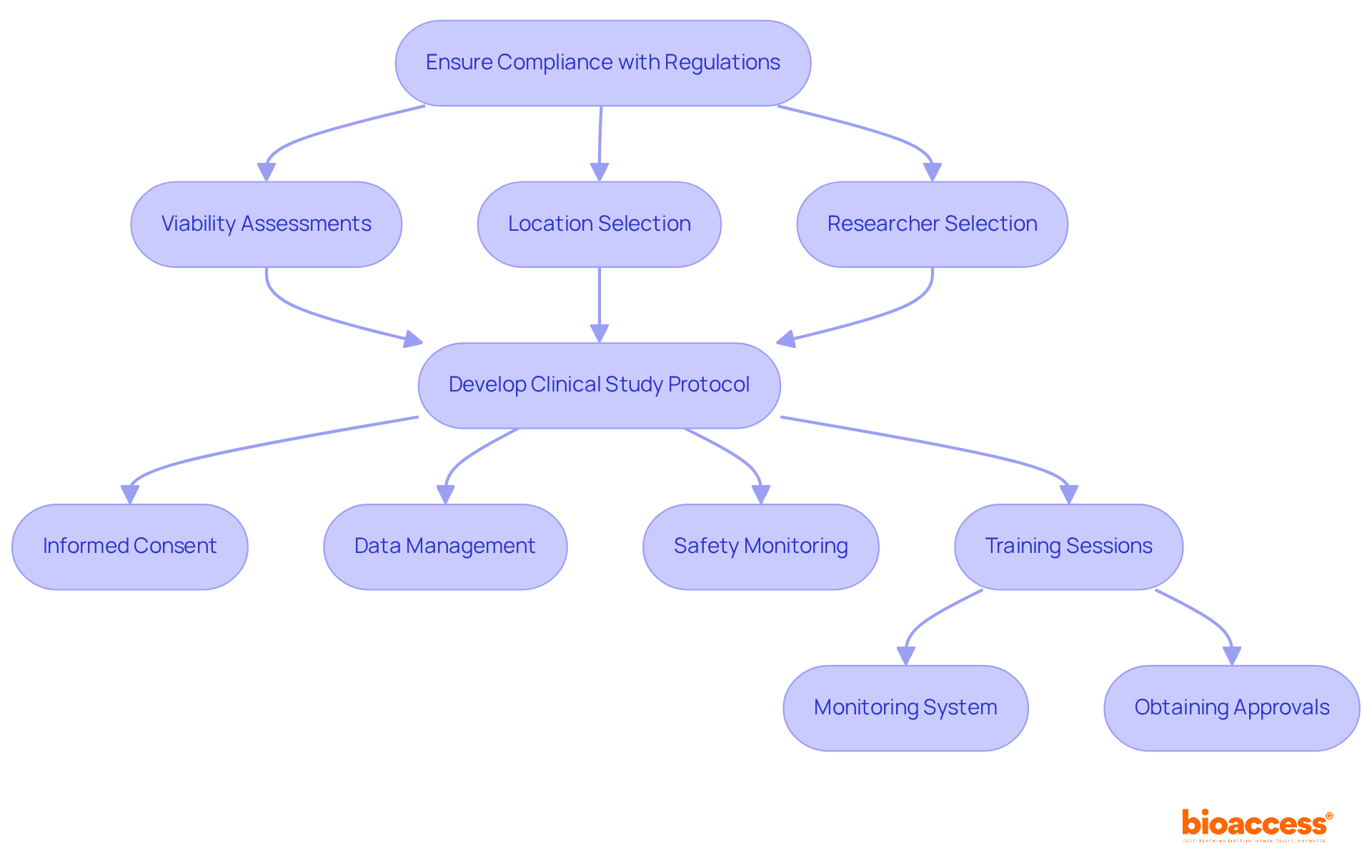
Understanding the regulatory environment is essential for executing successful studies involving human subjects. It is imperative to familiarize yourself with Good Clinical Practice (GCP) guidelines and local regulatory requirements, which have evolved significantly in 2025 to enhance participant safety and data integrity.
At bioaccess, we emphasize a comprehensive process that includes:
to ensure that your research efforts are poised for success. Develop a clinical study protocol that clearly delineates all procedures, encompassing:
Regular training sessions for your research team on compliance issues are vital, ensuring that everyone is well-versed in the latest regulations and ethical standards. Thorough documentation of all study-related activities is crucial for maintaining an audit trail and ensuring accountability.
Furthermore, establish a robust monitoring system to track compliance with protocols and swiftly address any deviations, thereby safeguarding the integrity of the research and enhancing the credibility of the trial results. Our services also encompass obtaining necessary approvals from ethics committees and health ministries, as well as managing import permits for investigational devices, ensuring that all regulatory hurdles are effectively navigated.
Upon completion of data collection, it is imperative to initiate the cleaning and organization of the data to guarantee accuracy.
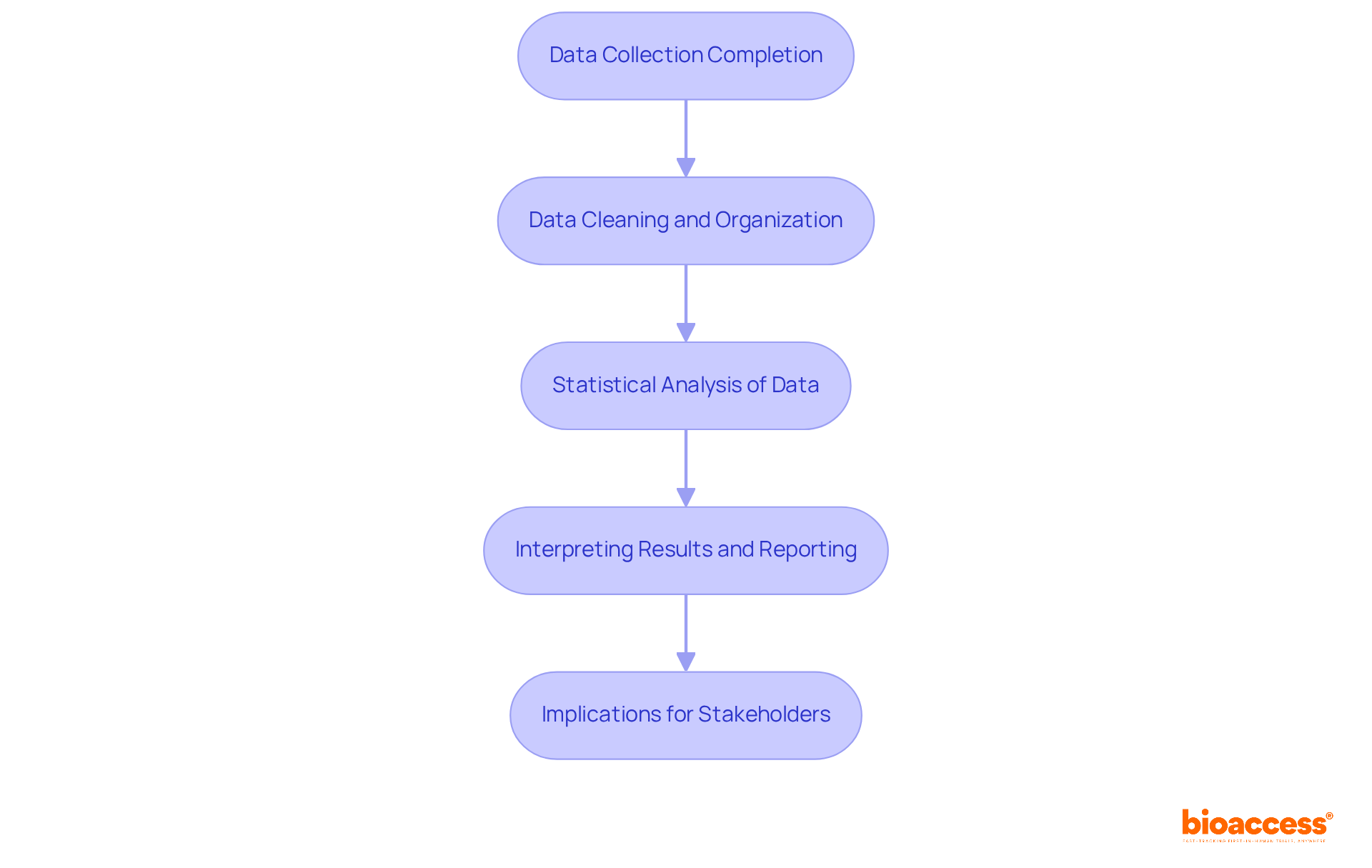
Planning successful pivotal studies in clinical research is a multifaceted process that requires meticulous attention to various elements to achieve effective outcomes. A thorough understanding of the fundamentals of pivotal studies equips researchers to navigate the complexities inherent in Phase III trials, ultimately facilitating the validation of new medications and medical devices.
This article emphasizes the necessity of:
Each stage, from planning to execution, is critical to the overall success of the research. Engaging communities and leveraging strategic partnerships can significantly enhance participant recruitment and retention, while comprehensive training on compliance ensures that all regulatory standards are met.
In light of these insights, it is imperative for researchers to prioritize meticulous planning and execution of pivotal studies. By leveraging the strategies outlined and fostering collaboration within the research community, the path to successful clinical trials becomes increasingly clear. Embracing these practices not only advances scientific knowledge but also contributes to the development of safe and effective healthcare solutions for diverse populations.
What are pivotal studies, and why are they important?
Pivotal studies, also known as Phase III clinical evaluations, are essential for determining the effectiveness and safety of new medications or medical devices. They involve a substantial participant pool, typically ranging from 300 to 3,000 individuals, to generate robust data required for regulatory approval from organizations like the FDA and EMA.
What are the primary objectives of Phase III studies?
The primary objectives of Phase III studies include validating initial indications of safety and effectiveness gathered in earlier phases, assessing the drug's impact on quality of life, and analyzing its performance across diverse populations.
What types of outcomes are measured in pivotal studies?
Pivotal studies measure both primary outcomes, such as the drug's effectiveness, and secondary outcomes, which may include safety profiles and additional benefits.
How does the regulatory landscape affect pivotal studies?
Understanding the regulatory landscape and specific data requirements for pivotal studies is crucial, as it directly influences the planning and execution of successful research.
What factors should be considered when planning a pivotal study?
When planning a pivotal study, it is essential to clearly define research objectives, select a suitable research design, determine the sample size for statistical significance, and outline a well-organized protocol that includes participant selection criteria, data collection methods, and statistical analysis plans.
Why is sample size important in pivotal studies?
Sample size is crucial for achieving statistical significance, as phase III clinical trials typically involve several hundred patients to ensure robust data.
What role does bioaccess® play in pivotal studies?
Bioaccess® offers expertise in critical research areas, including regulatory approval and patient recruitment, which can enhance the efficiency of clinical trials by facilitating accelerated patient enrollment and achieving cohorts 50% faster than traditional methods.
How can incorporating validated instruments and measures benefit pivotal studies?
Incorporating validated instruments and measures enhances the reliability of findings, as clarity in operational definitions of disease states and health outcomes is essential for good research practice.
What is the potential cost-saving benefit of leveraging bioaccess®'s services?
Leveraging bioaccess®'s services can potentially save $25K per patient, thus enhancing the overall efficiency of clinical trials.