


The article titled "7 Key Insights from Bio 2024 San Diego for Clinical Research Leaders" presents essential insights that clinical research leaders can derive from the Bio 2024 event. It underscores the importance of leveraging research agility in Latin America, highlights the transformative role of AI in biotech operations, and stresses the necessity of prioritizing patient impact in oncology research to enhance treatment outcomes and streamline clinical studies.
The landscape of clinical research is rapidly evolving, driven by innovations and insights that redefine how studies are conducted and how patient needs are prioritized. As leaders in the field gather for Bio 2024 in San Diego, they are presented with key strategies that not only enhance operational efficiency but also emphasize the importance of agility and technology in research.
How can clinical research leaders harness these insights to overcome existing challenges and accelerate the development of groundbreaking therapies?
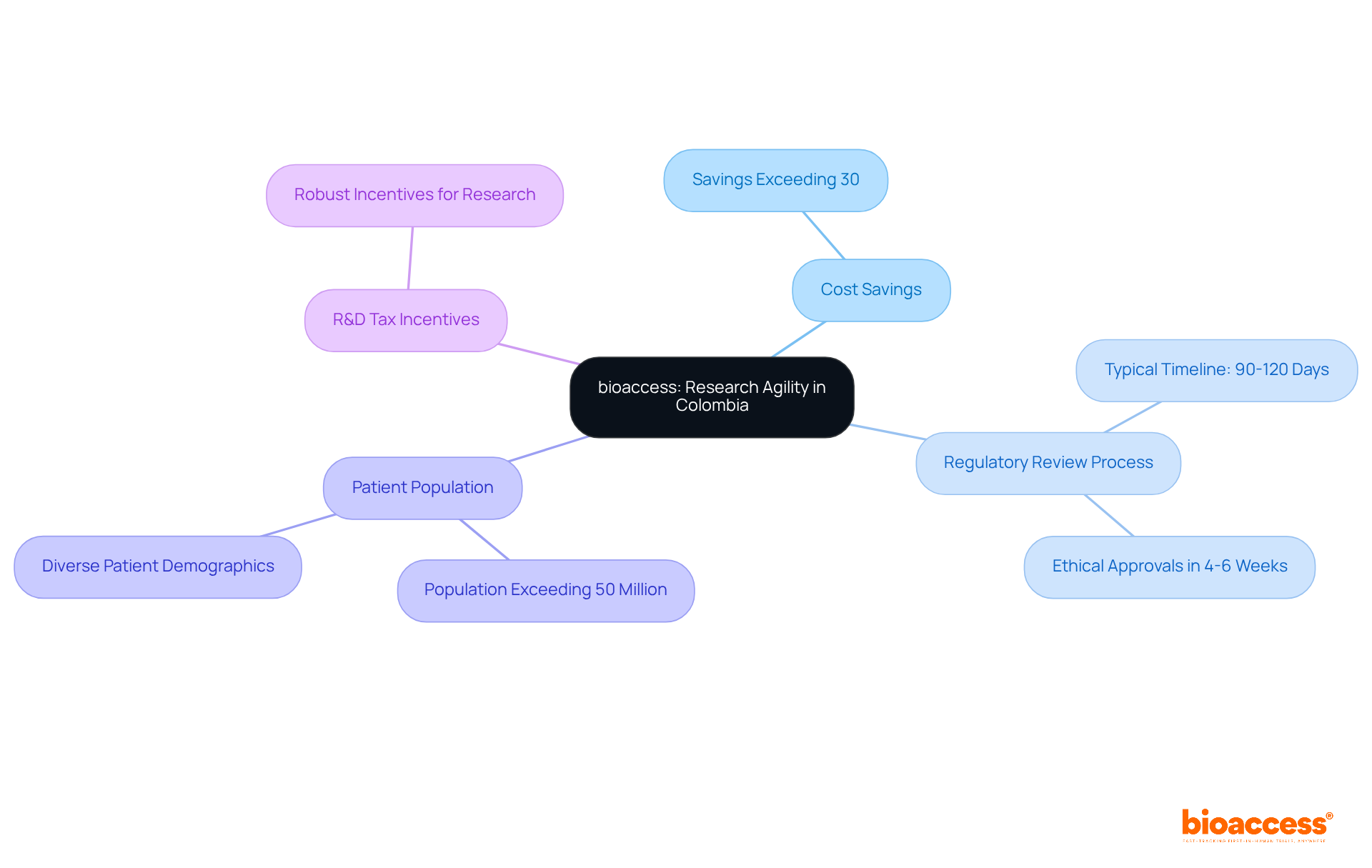
bioaccess® strategically leverages its position in Latin America, particularly in Colombia, to deliver unparalleled research agility. With cost savings exceeding 30% compared to North America and Western Europe, Colombia also boasts a regulatory review process that typically spans only 90-120 days. By capitalizing on local regulatory advantages and diverse patient populations, bioaccess® can secure ethical approvals in a mere 4-6 weeks—significantly faster than in traditional markets.
This capability empowers Medtech, Biopharma, and Radiopharma innovators to initiate studies and gather data more rapidly, thereby accelerating the path to commercialization. This agility not only enhances operational efficiency but also fosters innovation in medical study methodologies, positioning it as a vital factor for leaders in the field.
Furthermore, with a population exceeding 50 million and a healthcare system recognized among the best globally, Colombia emerges as a prime location for medical studies, further strengthened by robust R&D tax incentives that enhance its appeal.
Artificial intelligence is revolutionizing the biotech landscape by streamlining operations and enhancing research capabilities. This technology is transforming how studies are conducted, from predictive analytics that improve study designs to AI-driven participant recruitment strategies.
For instance, bioaccess® enables treatment-naive cardiology or neurology groups to be enrolled 50% quicker than conventional Western sites, achieving substantial savings of $25K per individual with FDA-ready data—no rework, no delays. This innovative method not only shortens enrollment durations but also addresses the typical obstacles faced by Medtech and Biopharma startups in early-stage studies.
Dr. Jane Smith, a Clinical Research Management Expert, emphasizes that embracing AI alongside bioaccess®'s expert services enhances operational efficiency while ensuring compliance with regulatory standards. This ultimately leads to improved outcomes for individuals and expedited drug development cycles.
Furthermore, bioaccess® connects cutting-edge Medtech, Biopharma, and Radiopharma startups with leading clinical sites, providing a comprehensive solution for accelerating clinical studies.

In oncology, addressing unmet healthcare needs is paramount for advancing treatment options and improving outcomes. Clinical research leaders must prioritize participant involvement throughout the study process, from design to execution. This entails comprehending the particular challenges encountered by individuals with cancer and integrating their feedback into research protocols. Trials that focus on patient-reported outcomes provide valuable insights into treatment efficacy and tolerability. The Clinical Research Management Expert emphasizes that by centering research around patient needs, organizations can enhance enrollment and retention rates, while also contributing to the development of therapies that truly address the challenges faced by patients in their cancer journeys.

The insights gathered from Bio 2024 San Diego underscore the transformative potential of strategic agility and innovation in clinical research. By leveraging unique advantages in regions like Colombia and embracing cutting-edge technologies such as artificial intelligence, clinical research leaders can significantly enhance operational efficiencies and accelerate the path to market for new therapies.
Key discussions highlighted the importance of bioaccess® in facilitating faster regulatory approvals and patient recruitment, which are crucial for Medtech and Biopharma innovators. Additionally, prioritizing patient impact, particularly in oncology, emerged as a critical factor in designing studies that truly address the needs of individuals facing serious health challenges. This patient-centric approach not only improves outcomes but also fosters a more inclusive and effective research environment.
Reflecting on these insights, it is evident that the future of clinical research lies in a collaborative and adaptive framework that prioritizes speed, efficiency, and patient engagement. As the landscape continues to evolve, embracing these principles will be essential for leaders aiming to drive meaningful advancements in healthcare and improve the lives of patients globally.
What is bioaccess® and what does it offer for clinical research?
bioaccess® is a company that leverages its strategic position in Latin America, particularly Colombia, to enhance research agility, offering cost savings and faster regulatory processes for clinical studies.
How much cost savings can be expected when conducting research in Colombia compared to North America and Western Europe?
Cost savings can exceed 30% when conducting research in Colombia compared to North America and Western Europe.
What is the typical duration of the regulatory review process in Colombia?
The regulatory review process in Colombia typically spans only 90-120 days.
How quickly can bioaccess® secure ethical approvals for studies in Colombia?
bioaccess® can secure ethical approvals in a mere 4-6 weeks, which is significantly faster than in traditional markets.
What advantages does Colombia offer for Medtech, Biopharma, and Radiopharma innovators?
Colombia offers diverse patient populations, a fast regulatory process, and cost efficiencies, allowing innovators to initiate studies and gather data more rapidly, thereby accelerating commercialization.
What is the population size of Colombia and how does this impact clinical research?
Colombia has a population exceeding 50 million, providing a large and diverse participant pool for medical studies, which enhances the potential for robust clinical research.
How is Colombia's healthcare system regarded globally?
Colombia's healthcare system is recognized among the best globally, making it an attractive location for conducting medical studies.
Are there any financial incentives for conducting research in Colombia?
Yes, Colombia offers robust R&D tax incentives that enhance its appeal for conducting medical studies.