


The article delineates seven strategies for achieving clinical research success within the context of Bio 2024, underscoring the significance of leveraging regional advantages, innovative technologies, and collaborative partnerships. These strategies encompass:
Collectively, these efforts aim to reduce costs while improving the efficiency and outcomes of clinical trials.
The landscape of clinical research is evolving rapidly, characterized by innovative strategies that enhance efficiency and effectiveness in study execution. As organizations gear up for Bio 2024, grasping the key factors that contribute to successful clinical trials is essential.
What best practices can streamline processes while ensuring robust patient engagement and diverse representation in studies? This article delves into seven pivotal strategies poised to reshape clinical research success, providing insights into how companies can leverage these approaches to navigate the complexities of the healthcare environment and achieve their research objectives.
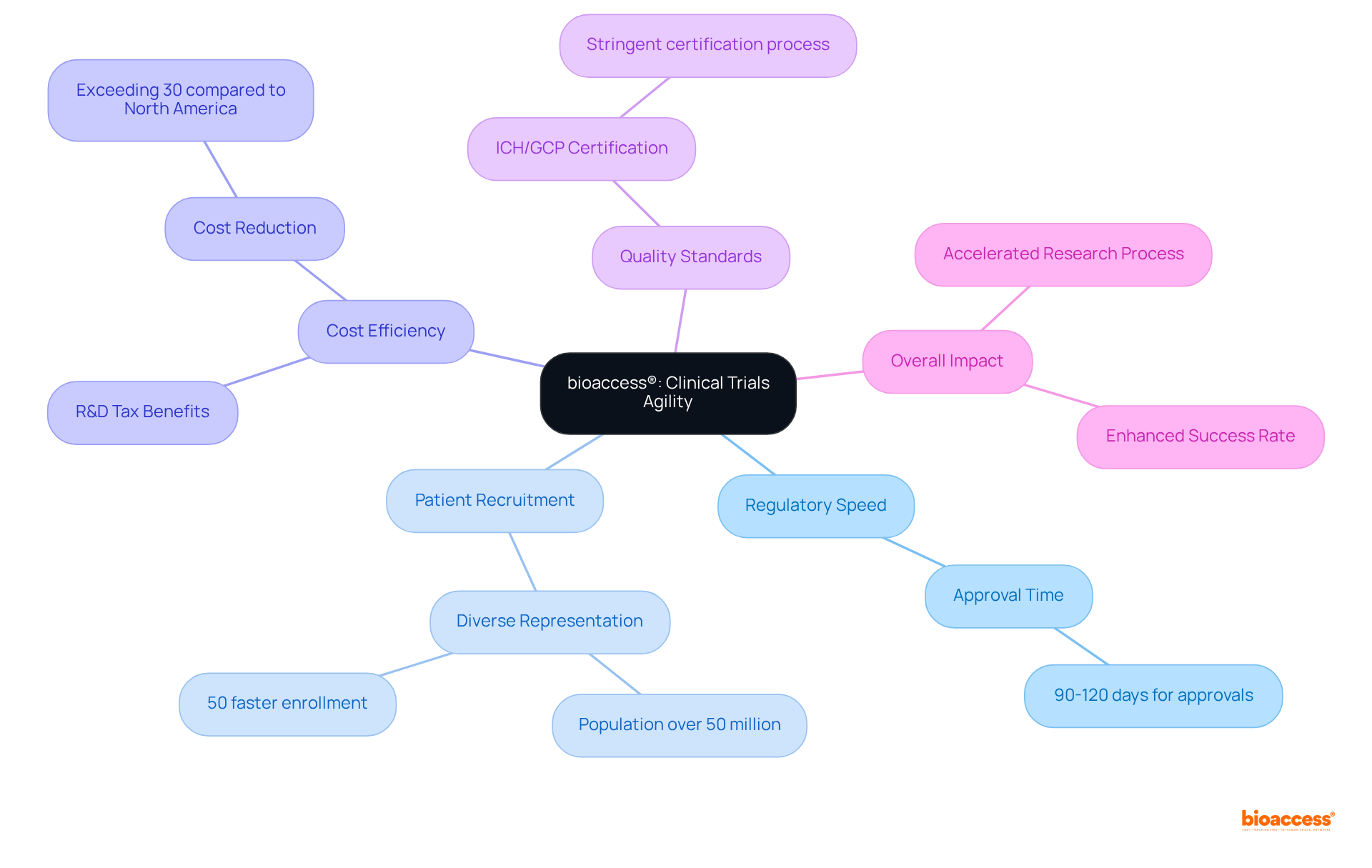
The company strategically leverages its sites in Latin America, particularly in Colombia, to deliver unparalleled speed in research execution. Colombia's regulatory advantages facilitate ethical approvals within a swift 90-120 days, significantly expediting the time to market for innovative medical technologies.
With a healthcare system recognized among the best globally and a population exceeding 50 million, bioaccess® effectively recruits patients for bio 2024, ensuring diverse representation in research studies.
Additionally, conducting experiments in Colombia yields cost reductions exceeding 30% compared to North America and Western Europe, complemented by R&D tax benefits that enhance the financial viability of studies.
Hospitals in Colombia undergo a stringent ICH/GCP certification process prior to conducting research, ensuring adherence to high-quality standards.
This agility not only accelerates the research process but also enhances the overall success rate of medical studies, establishing bioaccess® as a preferred partner for Medtech, Biopharma, and Radiopharma innovators.
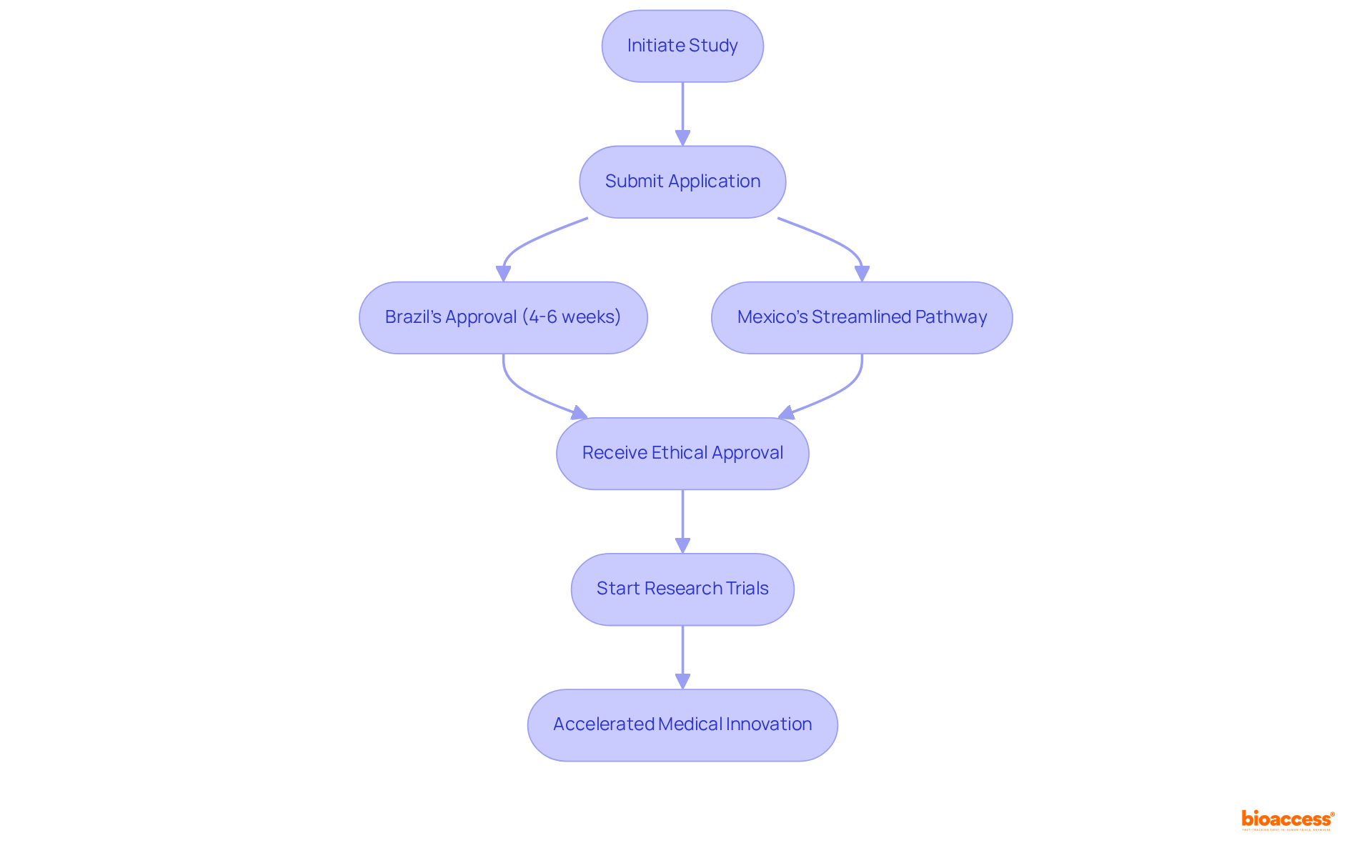
Latin America is distinguished by its swift regulatory procedures, enabling studies to secure authorizations significantly faster than in conventional markets. Brazil and Mexico have developed streamlined pathways that enhance the efficiency of submissions and reviews.
For instance, Brazil's regulatory framework allows for ethical approvals in just 4-6 weeks, while Mexico has made strides in reducing approval durations, making it an attractive destination for research trials.
By partnering with bioaccess® for bio 2024, companies can leverage these expedited approvals, ensuring that their research commences promptly. This not only accelerates the development of groundbreaking therapies but also facilitates quicker access to essential treatments for patients.
Successful studies in these countries exemplify the effectiveness of this approach, demonstrating how optimized procedures can lead to substantial advancements in medical innovation and patient care.
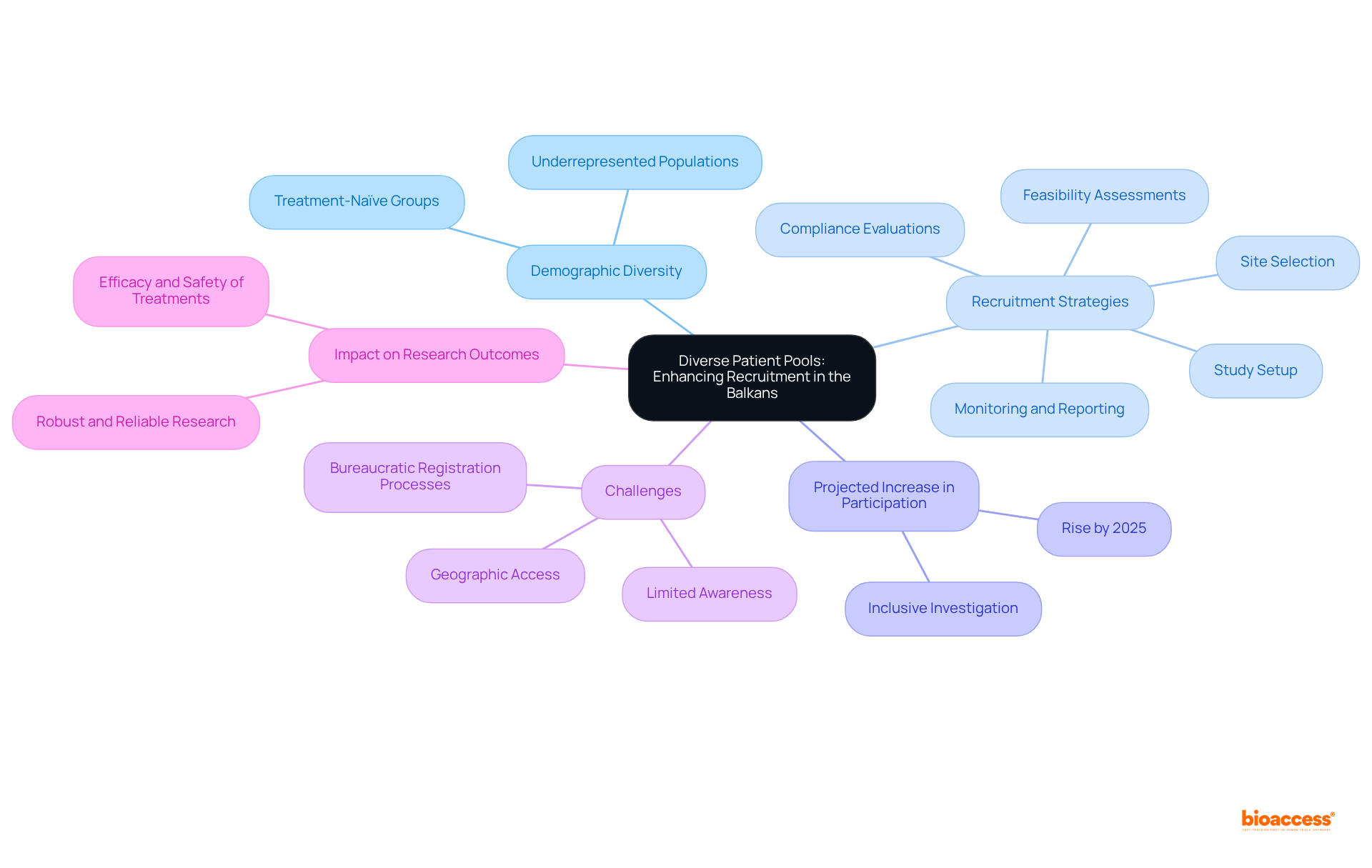
The Balkans present a diverse demographic landscape, crucial for engaging treatment-naïve groups that are frequently underrepresented in research studies. This diversity not only enhances recruitment efforts but also ensures that study findings are relevant to a wider audience.
By 2025, the proportion of treatment-naïve patients participating in research studies in the Balkans is projected to rise, highlighting the region's potential for inclusive investigation.
Leveraging bioaccess's comprehensive study management services—including feasibility assessments, site selection, compliance evaluations, study setup, import permits, project management, monitoring, and reporting—sponsors can significantly bolster their recruitment strategies for bio 2024. This ultimately leads to more robust and reliable research outcomes.
The focus on varied demographics is vital, as it contributes to the overall efficacy and safety of treatments across different populations, paving the way for improved healthcare solutions for all.
Nonetheless, challenges such as geographic access and limited awareness may impede participation, an issue that bioaccess can effectively address through targeted strategies.
As Dr. Freda Lewis-Hall noted, diversity in clinical studies is essential to ensure that every patient has the best opportunity for effective treatment.
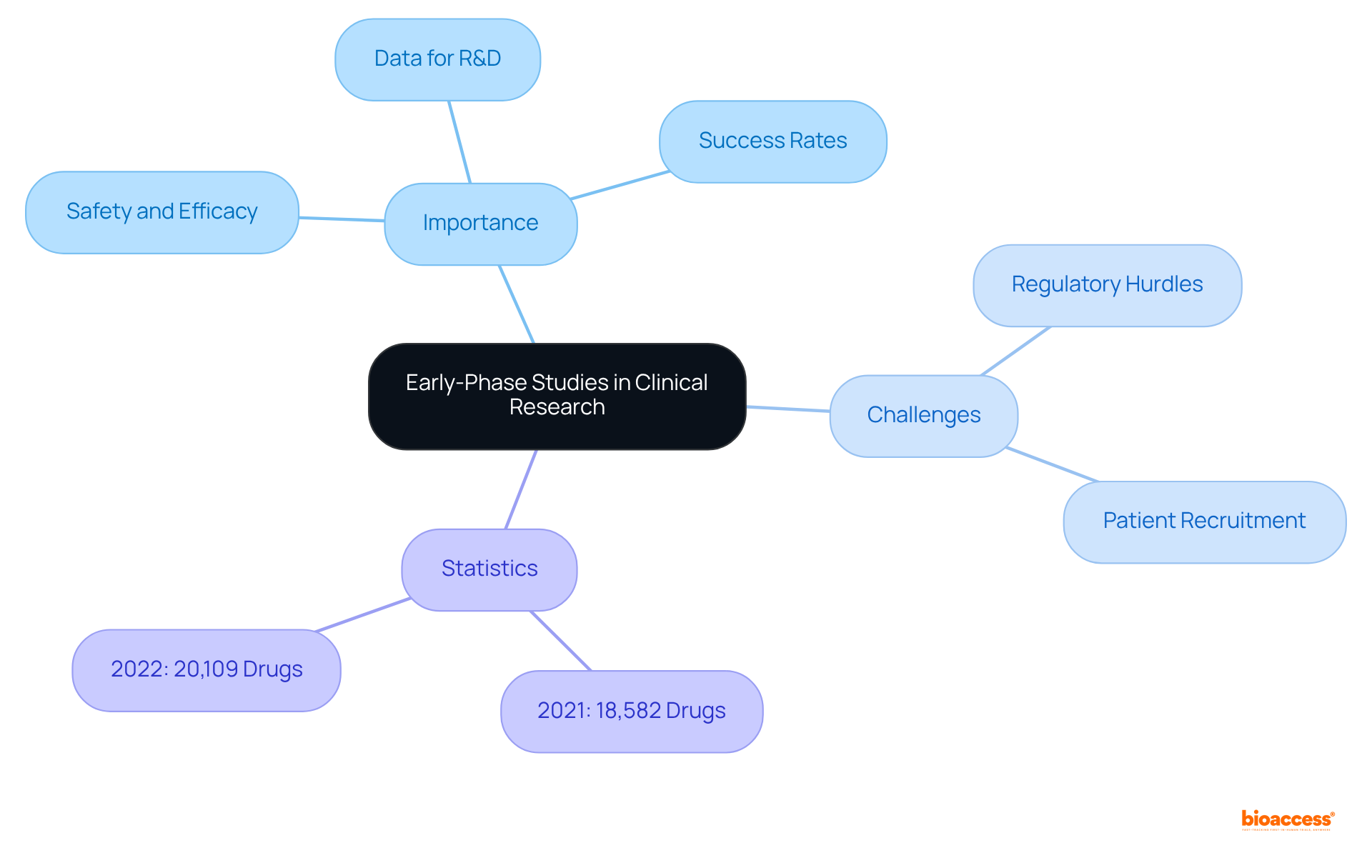
Early-phase studies, particularly first-in-human (FIH) and early-feasibility assessments, play a pivotal role in evaluating the safety and efficacy of innovative medical technologies. These trials yield essential data that inform subsequent research and development phases, ultimately shaping the success of new treatments. With over 15 years of experience, bioaccess® stands out in conducting these early-phase studies, ensuring they are carried out with both efficiency and ethical integrity. This approach not only accelerates the research timeline but also enhances the likelihood of successful outcomes.
Nonetheless, early-phase clinical studies frequently encounter obstacles due to intricate regulatory requirements and challenges in patient recruitment. The approval process can be protracted and perplexing, presenting significant barriers for startups within the medtech and biopharma sectors. The system effectively addresses these hurdles through its streamlined processes and expert guidance, facilitating a more seamless approval experience. Dushyanth Surakanti, Founder and CEO of Sparta Biomedical, recounted his positive experience with the product during its initial human study in Colombia, highlighting its adept navigation of these challenges.
Significantly, the number of drugs in the R&D pipeline has surged, climbing from 18,582 in 2021 to over 20,109 as of 2022, indicative of ongoing advancements in medical research. The critical role of FIH trials is underscored by their function in establishing a robust foundation for future clinical research, rendering them indispensable in the journey from innovation to commercialization.
The company offers essential market access services, empowering clients to navigate the complex landscape of product launches in Latin America. With a deep understanding of local market dynamics and regulatory frameworks, bioaccess® provides companies with tailored strategies for successful commercialization in bio 2024. This support is crucial, as the region poses unique challenges, including the necessity for strict compliance with the 510(k) process for Class 2 medical devices, which represent 43% of all healthcare items in the U.S.
Notably, Colombia presents significant advantages for clinical studies, with cost reductions exceeding 30% compared to North America or Western Europe, alongside a regulatory review process that typically requires only 90-120 days. Furthermore, Colombia's healthcare system is highly regarded, ranked #22 by the World Health Organization, and approximately 95% of its population benefits from universal healthcare, facilitating participant recruitment.
Additionally, investments in R&D can capitalize on substantial tax incentives, enhancing the attractiveness of conducting experiments in Colombia. By leveraging its expertise, the organization guarantees that innovative medical technologies effectively reach the patients who need them most, thereby maximizing their potential impact on public health within the bio 2024 multi-billion dollar healthcare market.
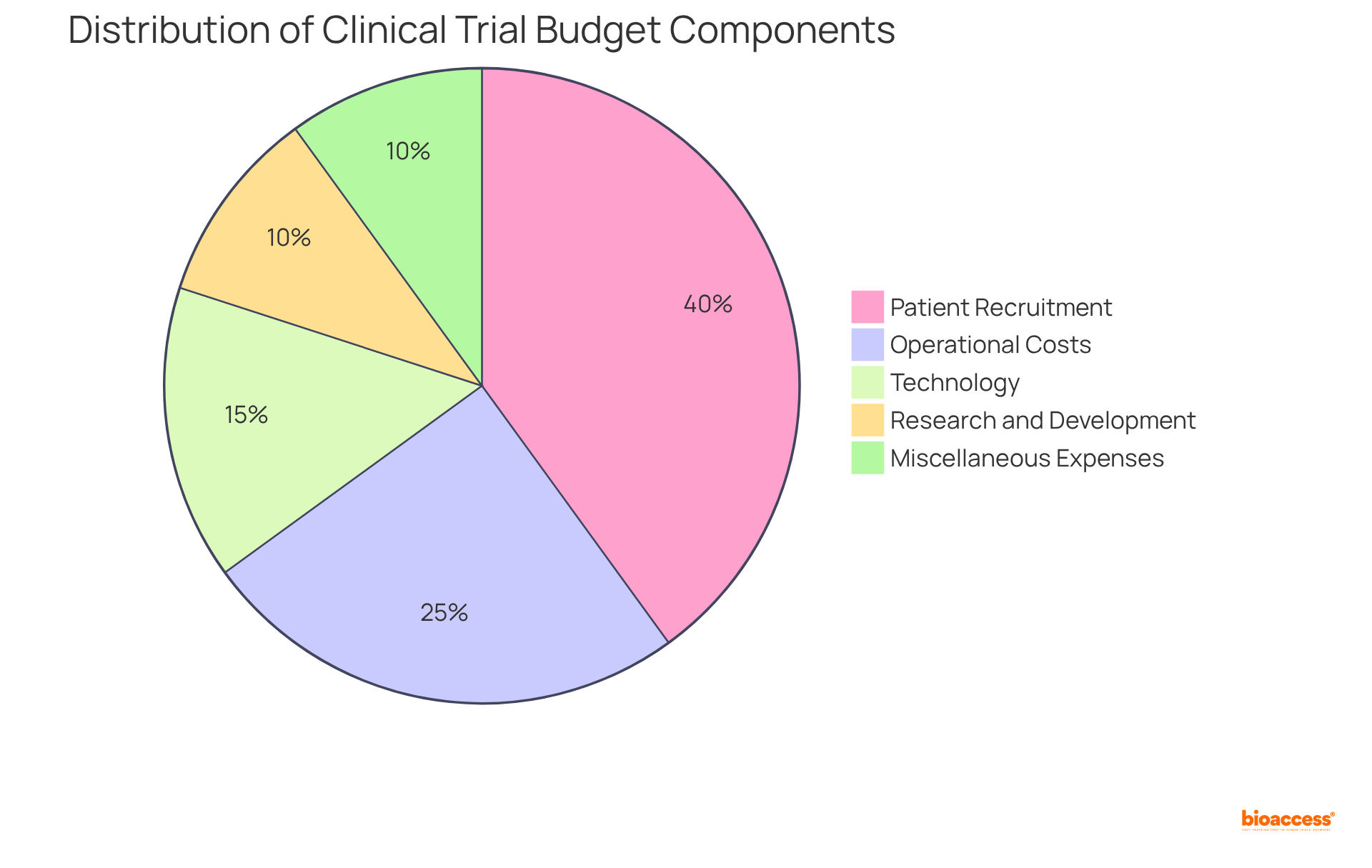
Carrying out clinical studies frequently presents significant financial challenges. However, bio 2024 offers innovative solutions that enhance research budgets. By leveraging local resources and expertise, the organization effectively reduces operational costs without compromising quality. This strategic approach empowers clients to allocate their budgets more efficiently, directing funds toward essential areas of research and development. The integration of sophisticated budgeting tools and streamlined processes can yield substantial savings, facilitating a more concentrated investment in critical components of the study.
As we look to 2025, optimizing research budgets will be crucial, particularly given that the projected cost of patient recruitment alone accounts for approximately 40% of the overall trial budget, totaling around $1.89 billion. By prioritizing budget allocation and harnessing technology, bioaccess not only enhances financial efficiency but also supports the successful advancement of research studies for bio 2024. This collaboration is vital in navigating the complexities of the Medtech landscape and addressing the key challenges faced in clinical research.
Regulatory compliance is paramount in medical research, as it safeguards ethical conduct and prioritizes participant safety. bioaccess® rigorously adheres to regulatory standards, instilling confidence in clients that their studies will align with necessary guidelines. This unwavering commitment not only protects participants but also enhances the credibility of research findings.
Organizations that implement robust compliance frameworks can save an average of $1.08 million, exemplifying the financial advantages of maintaining high ethical standards. Furthermore, research trials employing integrated data management systems demonstrate a 30% faster enrollment of participants and a 45% quicker database lock, underscoring the efficiency achieved through compliance.
As emphasized by industry specialists, regulatory adherence transcends being merely an obstacle; it represents a continuous dedication to patient safety and product quality, which is vital for fostering trust in research.
The medical research landscape is undergoing a significant transformation, driven by the integration of innovative technologies that are reshaping healthcare delivery. The company is at the forefront of this evolution, actively incorporating cutting-edge technologies into its research methodologies. This dedication to innovation not only simplifies research procedures but also plays an essential part in the creation of pioneering therapies that have the potential to transform healthcare.
bioaccess® allows treatment-naive cardiology or neurology groups to be enrolled 50% quicker than conventional Western sites, realizing substantial cost savings of $25K per individual with FDA-ready data—removing rework and delays. This efficiency is essential in tackling the typical obstacles encountered by Medtech and Biopharma startups in patient recruitment during early-stage studies.
Recent statistics show that around 80% of research studies now employ Electronic Data Capture (EDC) systems, reflecting a significant shift towards digital solutions in study management. Furthermore, the adoption of decentralized clinical trials (DCTs) has surged, with 1,300 drug trials incorporating DCT components in 2022, marking a 28% increase from the previous year. These advancements enhance diversity and retention of individuals by allowing data collection from the comfort of their homes.
Thought leaders in the industry emphasize the importance of these technological advancements. Char Kumarage, Practice Lead in Clinical Research, notes that the COVID-19 pandemic catalyzed the adoption of DCTs, enabling patients to take a more active role in data collection. This change not only improves access for rural communities but also enhances the overall quality of medical research.
In partnership with Caribbean Health Group, the organization is also striving to establish Barranquilla as a premier location for medical research in Latin America, backed by Colombia's Minister of Health. As the company continues to utilize these innovations, it remains committed to enhancing study efficiency and outcomes for individuals, ensuring that the organization is well-prepared to address the changing requirements of the healthcare environment.
Adopting patient-focused strategies in clinical studies is essential for enhancing participant involvement and achieving better results. At bioaccess®, we prioritize the needs and desires of individuals throughout the research process for bio 2024, ensuring that study designs align with their expectations. This commitment fosters a collaborative environment, significantly enhancing patient satisfaction and retention.
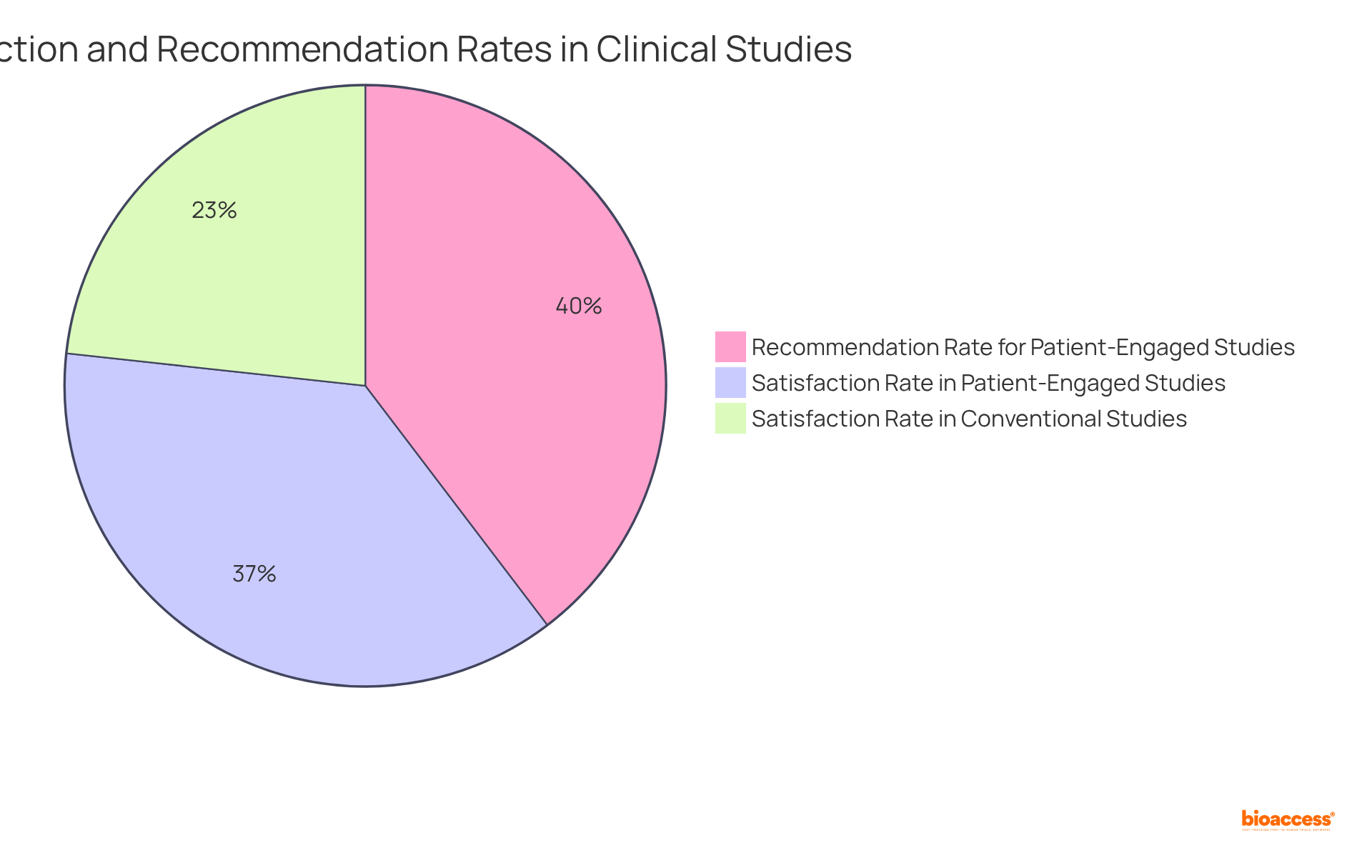
Research indicates that patient-involved recruitment strategies can shorten the average enrollment duration from 18 months to merely 12 months in oncology studies, illustrating a 23-34% quicker attainment of enrollment goals compared to conventional approaches. Moreover, 86% of participants in patient-engaged studies evaluate their experience as 'excellent', in contrast to only 54% in conventional studies. Additionally, 92% of participants would recommend involvement in the study to others when engagement is significant.
Recognizing obstacles early through participant feedback can result in a 67% decrease in recruitment timeline delays. By incorporating feedback from participants into trial designs, the organization not only aligns with participant priorities but also enhances overall trial outcomes, leading to more successful research findings.
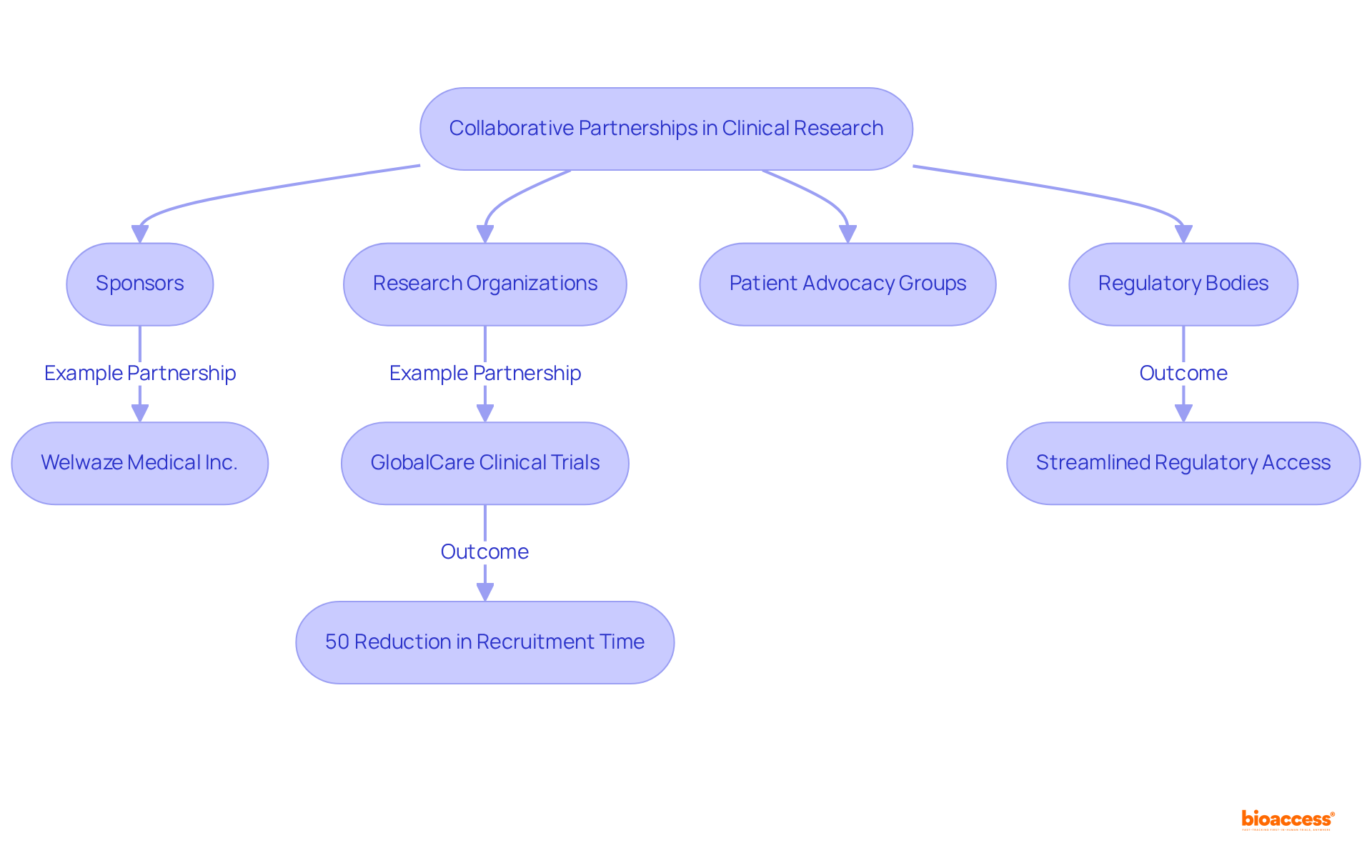
Collaborative partnerships are essential for achieving success in medical research. At bioaccess®, we prioritize the cultivation of strong relationships with stakeholders as part of our strategy for bio 2024, including sponsors, regulatory bodies, and patient advocacy groups. By enhancing resource sharing and facilitating knowledge exchange, we drive innovation that leads to more efficient and effective clinical studies. These partnerships not only improve trial outcomes but also advance the field of medical science.
For example, our collaboration with Welwaze Medical Inc. for the launch of the Celbrea® medical device in Colombia demonstrates how strategic partnerships can streamline regulatory access and market entry in Latin America. Additionally, our collaboration with GlobalCare Clinical Trials has resulted in an impressive reduction of over 50% in recruitment time and an outstanding 95% retention rate, underscoring the effectiveness of our expedited participant recruitment and site activation services.
Industry leaders emphasize that collaboration empowers researchers to leverage diverse expertise, ultimately accelerating the pace of innovation in medical research. The SENSCIS study further exemplifies this approach by employing a community advisory board to enhance patient safety and adapt procedures based on patient feedback, yielding more meaningful outcomes.
At bioaccess®, we are committed to nurturing these connections and providing comprehensive CRO trial services for bio 2024, ensuring that our trials not only succeed but also significantly contribute to the healthcare landscape. We invite you to explore how our collaborative approach can elevate your clinical research initiatives.
The strategies outlined for achieving clinical research success with bio 2024 underscore the transformative potential of innovative approaches within the medical research landscape. By leveraging unique regional advantages—such as expedited regulatory processes in Latin America and diverse patient recruitment strategies in the Balkans—organizations can significantly enhance the efficiency and effectiveness of their clinical trials.
Key insights from the article emphasize the importance of:
All of which contribute to a robust framework for successful research. Furthermore, the focus on regulatory compliance ensures that studies not only adhere to ethical standards but also foster trust and credibility in the findings. The integration of patient-centric approaches and collaborative partnerships further enhances participant engagement and drives meaningful outcomes, underscoring the interconnectedness of these strategies in achieving research objectives.
As the field of clinical research continues to evolve, embracing these strategies will be crucial for organizations seeking to navigate the complexities of the healthcare environment. By prioritizing innovation, collaboration, and patient engagement, stakeholders can ensure their studies not only meet regulatory requirements but also deliver impactful results that advance medical science and improve patient care. Exploring these avenues can pave the way for breakthroughs that transform healthcare delivery and accessibility, making it imperative for industry leaders to adopt these insights for a successful future in clinical research.
What is bioaccess® and what role does it play in clinical trials?
bioaccess® is a company that leverages its sites in Latin America, particularly in Colombia, to accelerate clinical trials by delivering fast research execution and facilitating ethical approvals within 90-120 days.
How does Colombia's healthcare system benefit clinical research?
Colombia has a recognized healthcare system and a population of over 50 million, which allows bioaccess® to effectively recruit a diverse range of patients for research studies, ensuring varied representation.
What are the cost advantages of conducting research in Colombia?
Conducting experiments in Colombia can lead to cost reductions exceeding 30% compared to North America and Western Europe, along with R&D tax benefits that improve the financial viability of studies.
What quality standards do hospitals in Colombia adhere to for research?
Hospitals in Colombia must undergo a stringent ICH/GCP certification process before conducting research, ensuring compliance with high-quality standards.
What are the regulatory advantages of conducting studies in Latin America?
Latin America features swift regulatory procedures, allowing for faster authorizations compared to conventional markets. For example, Brazil offers ethical approvals in just 4-6 weeks, while Mexico has also improved its approval durations.
How does bioaccess® facilitate quicker access to treatments?
By partnering with bioaccess® for bio 2024, companies can take advantage of expedited regulatory approvals, allowing their research to commence promptly and enabling quicker access to essential treatments for patients.
What demographic advantages does the Balkans region offer for clinical studies?
The Balkans have a diverse demographic landscape that is crucial for engaging treatment-naïve groups, which are often underrepresented in research studies, enhancing recruitment efforts and the relevance of study findings.
How does bioaccess® support recruitment strategies in the Balkans?
bioaccess® provides comprehensive study management services, including feasibility assessments, site selection, compliance evaluations, and project management, which can significantly improve recruitment strategies for research studies.
What challenges might affect patient participation in studies in the Balkans?
Geographic access and limited awareness may impede patient participation in studies, but bioaccess® can address these issues through targeted strategies.
Why is diversity in clinical studies important?
Diversity in clinical studies is essential to ensure that treatments are effective across different populations, ultimately leading to improved healthcare solutions for all patients.