


The primary advantages of the FDA's Fast Track Designation for drug development encompass:
These factors collectively contribute to a significant reduction in the time to market for innovative therapies. Notably, this designation accelerates the approval timeline by as much as 15 months. Furthermore, it fosters collaboration among stakeholders, ultimately leading to quicker access to life-saving treatments for patients in need.
The pharmaceutical landscape is evolving rapidly, with the Fast Track Designation from the FDA emerging as a transformative strategy for drug development. This designation not only accelerates the approval process but also enhances communication between developers and regulatory bodies, ultimately benefiting patients who require timely access to innovative therapies. However, as companies race to leverage these advantages, a critical question arises: how can they effectively navigate the complexities of this expedited pathway to maximize their chances of success?
bioaccess® excels in simplifying the Rapid Pathway process for pharmaceutical development, drawing upon over 20 years of experience navigating intricate regulatory landscapes across Latin America, the Balkans, and Australia. This strategic approach empowers Medtech, Biopharma, and Radiopharma innovators to secure ethical approvals in an impressive 4-6 weeks.
With more than 50 pre-qualified sites activated in under 8 weeks and FDA/EMA/MDR-ready datasets featuring centralized monitoring, bioaccess® guarantees rapid site activation and a 50% faster enrollment rate compared to traditional markets. This positions bioaccess® as an essential partner for companies aiming to achieve fast track designation FDA for their therapies' journey to market.
The impact of Rapid Pathway Status on medication development timelines is substantial; it not only expedites the approval process but also enhances the likelihood of successful marketing, ultimately aiding patients in need of innovative therapies.
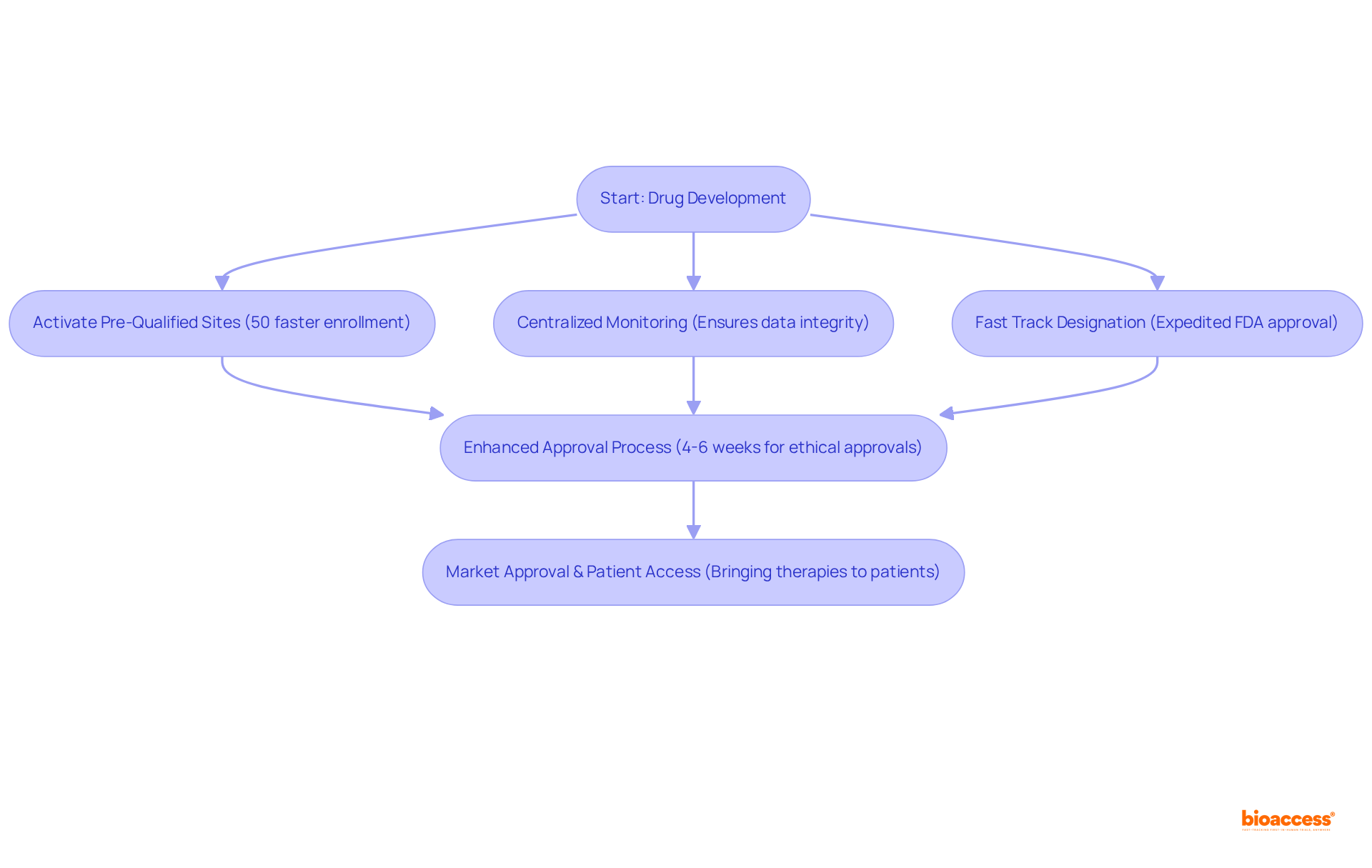
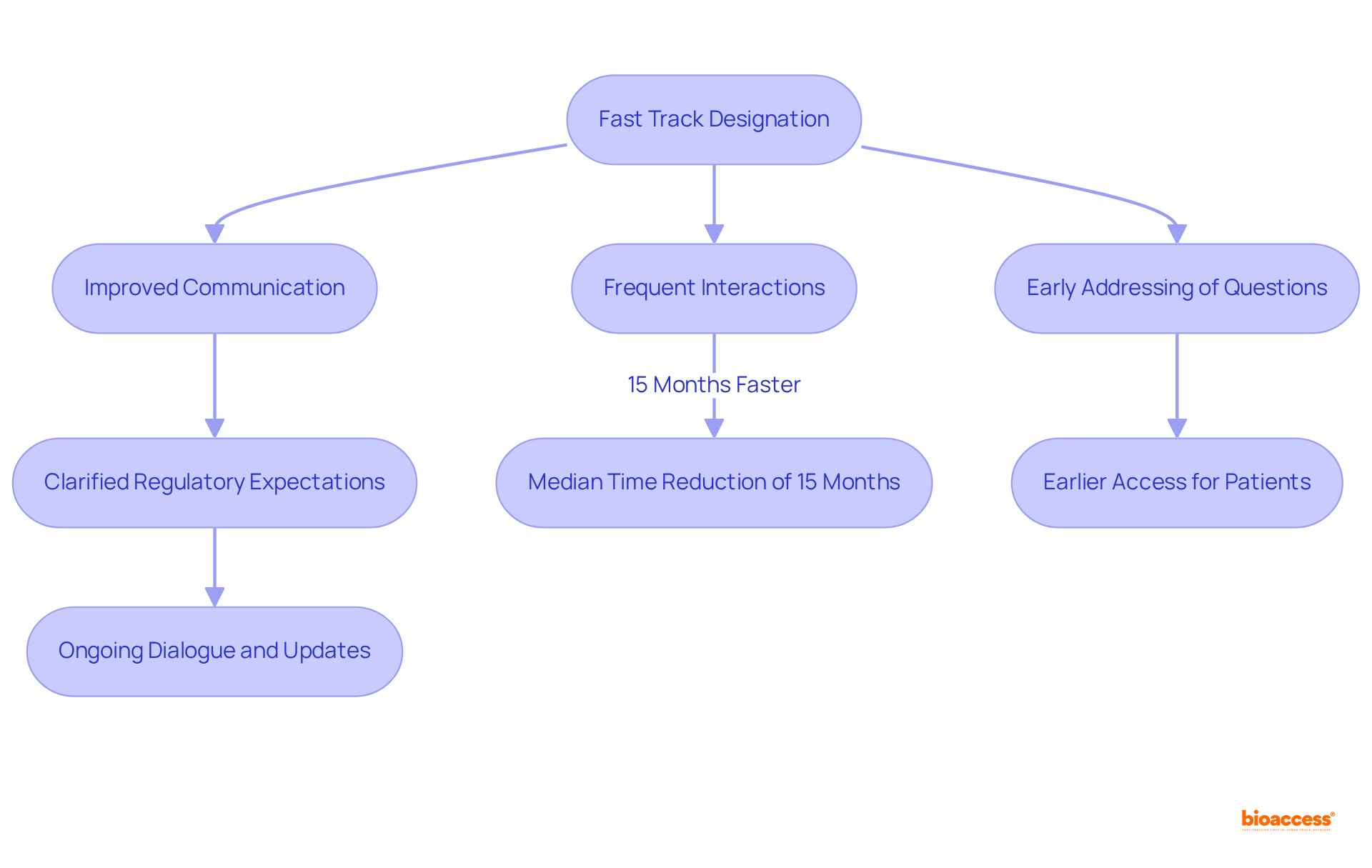
A notable benefit of the fast track designation FDA is the improved communication it fosters between medication developers and the FDA. This fast track designation FDA allows for more frequent interactions, which enables sponsors to address questions and concerns early in the development process. Such proactive engagement clarifies regulatory expectations and can lead to a median time reduction of 15 months in the approval process, particularly when seeking fast track designation FDA compared to industry benchmarks. This acceleration is crucial for patients awaiting new therapies, as the fast track designation from the FDA facilitates earlier access to potentially life-saving treatments. Moreover, medications with Accelerated designation receive regular updates and continuous evaluations, ensuring that any data deficiencies are recognized and resolved swiftly. This ongoing dialogue not only simplifies the approval process but also underscores the significance of teamwork in navigating the intricacies of medication development.
At bioaccess®, we specialize in regulatory approval, clinical research site activation, and patient recruitment, connecting innovative Medtech, Biopharma, and Radiopharma startups with top-ranked clinical research sites. This ensures that your clinical trials can start 40% faster and navigate the complexities of regulatory approval with confidence. To learn more about how we can assist you in expediting your clinical trials, contact us today.
The Rapid Pathway title significantly accelerates the evaluation process for medications targeting severe conditions. This approach facilitates rolling reviews, allowing portions of the application to be submitted and reviewed as they are completed, enabling the FDA to provide feedback more swiftly. Such a streamlined method not only shortens the overall approval timeline but also permits timely adjustments based on FDA input, thereby enhancing the likelihood of successful outcomes.
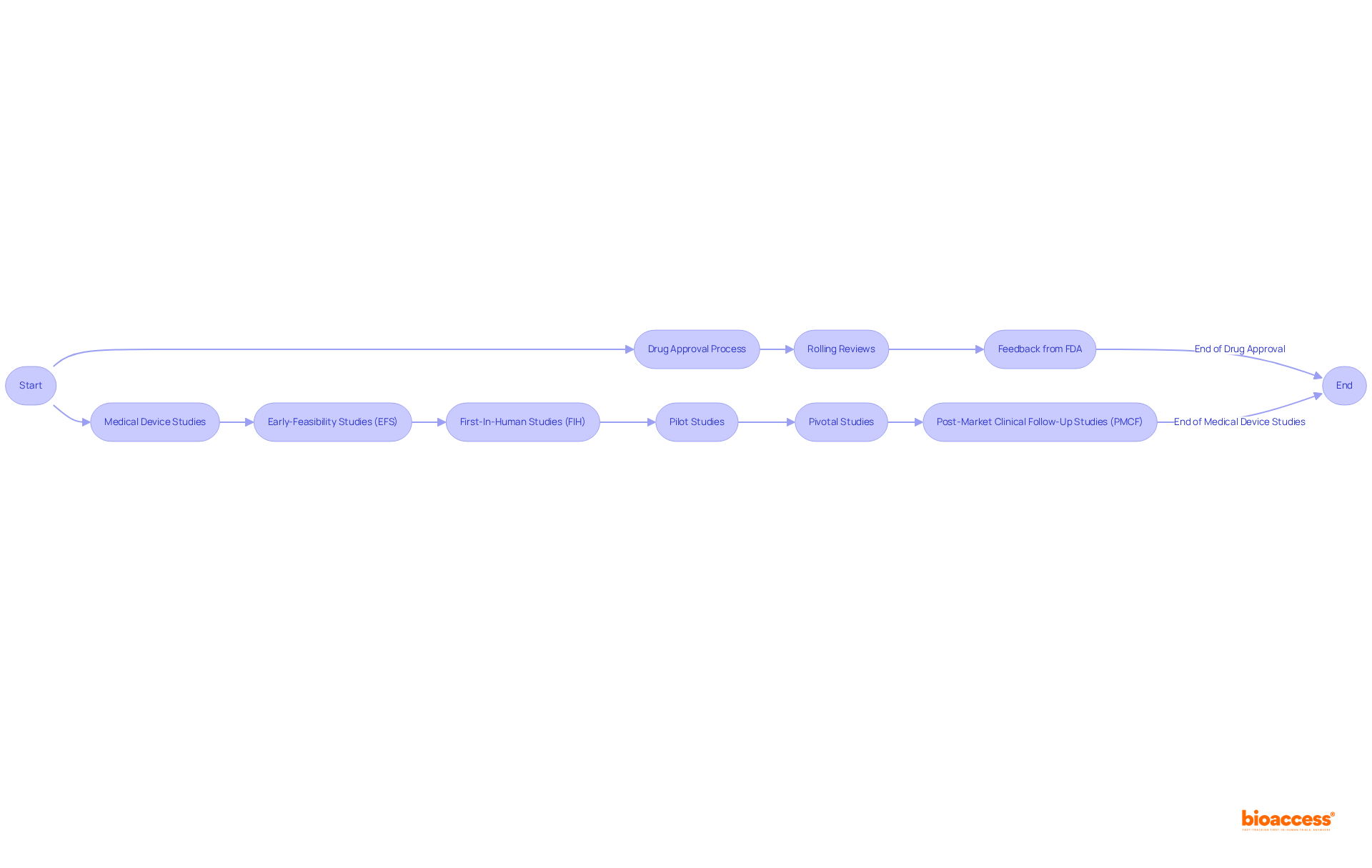
In the realm of medical devices, expedited processes can similarly be achieved by leveraging the expertise of bioaccess, which specializes in managing:
By implementing a tailored strategy for clinical trials, bioaccess can navigate the complexities of regulatory pathways, potentially reflecting the efficiencies observed in medication approvals.
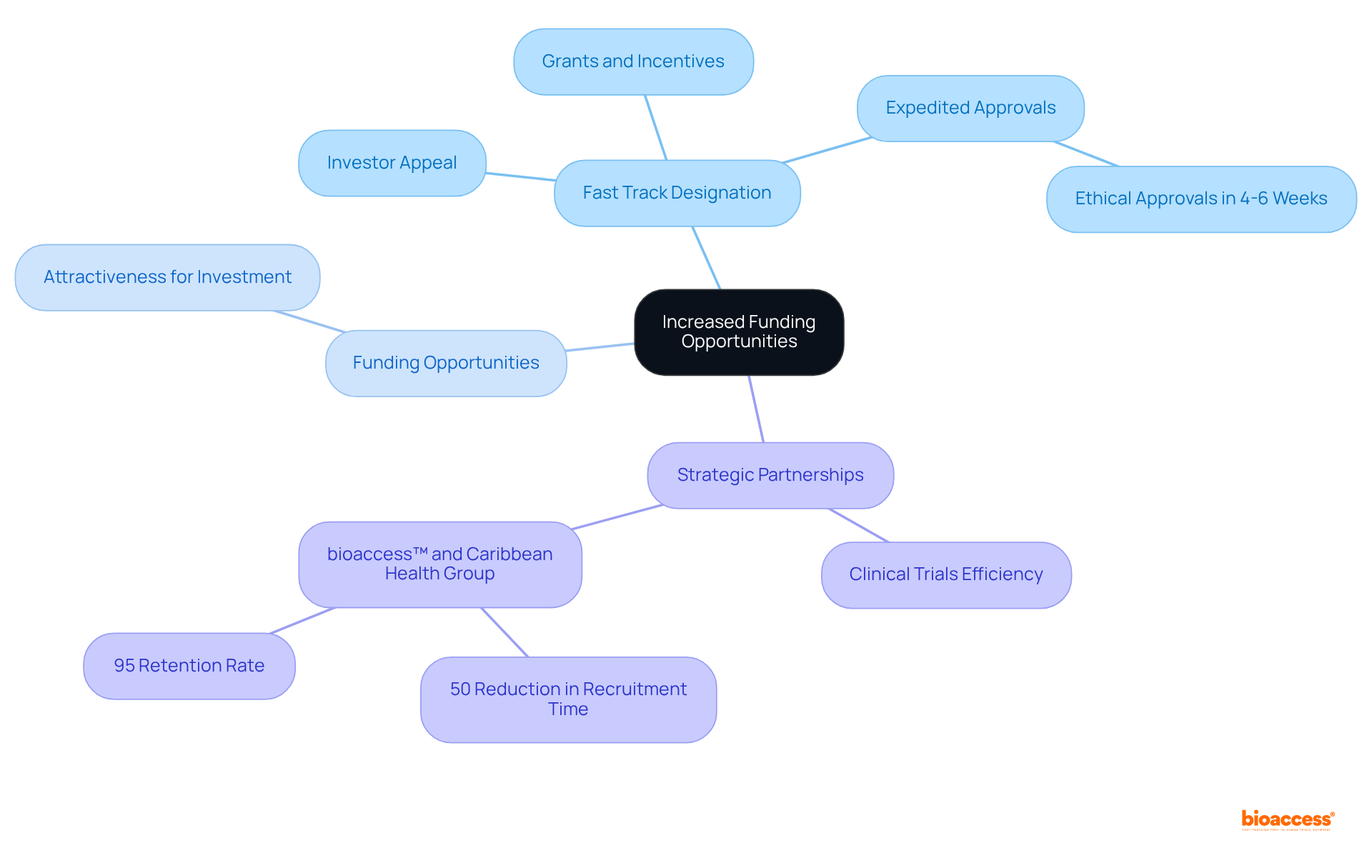
Securing fast track designation FDA significantly enhances funding opportunities for pharmaceutical developers. This classification, known as the fast track designation FDA, is frequently viewed by investors and funding organizations as a crucial indicator of a medication's potential success, thereby increasing the attractiveness of companies for investment. Furthermore, specific grants and financial incentives are often available for treatments that address unmet medical needs, improving the financial feasibility of these projects. With ethical approvals delivered in just 4-6 weeks, this expedited process not only accelerates development but also positions companies favorably in the eyes of investors.
The collaboration between bioaccess™ and Caribbean Health Group to position Barranquilla as a leading destination for clinical trials in Latin America, supported by Colombia's Minister of Health, exemplifies how strategic partnerships can enhance clinical trial efficiency. This initiative has already resulted in a remarkable over 50% reduction in recruitment time and a 95% retention rate, showcasing the potential for increased funding opportunities in a thriving clinical research environment.
The fast track designation FDA can lead to priority review status, significantly reducing the standard review time for New Drug Applications (NDAs) and Biologics License Applications (BLAs) from 10 months to just 6 months. This expedited timeline, supported by fast track designation FDA, enables companies to bring their products to market faster than competitors, which is crucial in therapeutic areas with high unmet needs. By securing priority review, companies position themselves as leaders in their markets, potentially capturing substantial market share early on.
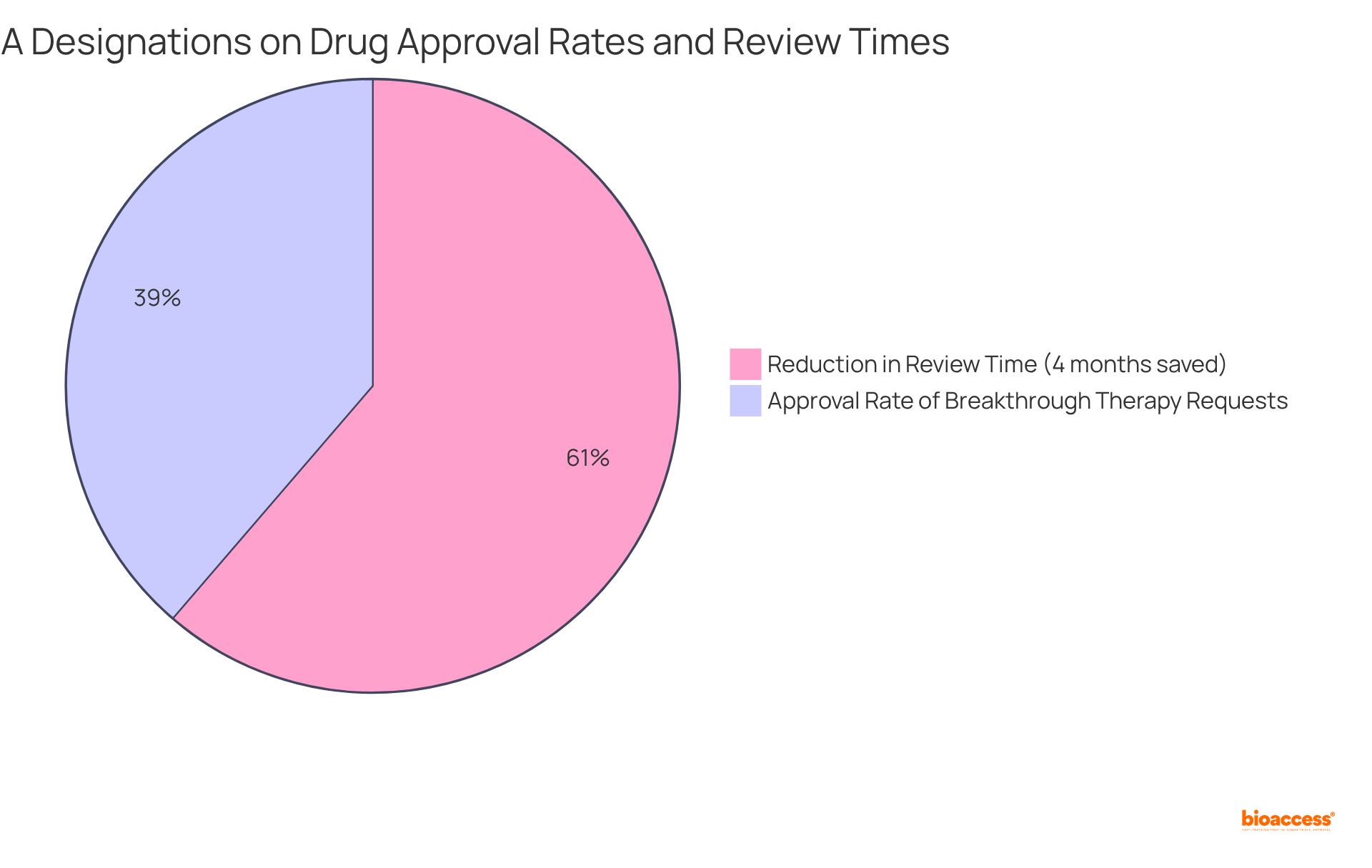
Medications with Breakthrough Therapy classification have shown an estimated 30% decrease in clinical development duration compared to non-designated medications, highlighting the competitive advantage that accelerated pathways offer. As of mid-2024, around 38.7% of Breakthrough Therapy requests have been approved, emphasizing the growing acknowledgment of the benefits these classifications provide in medication development. Companies that effectively leverage the fast track designation FDA can not only accelerate their timelines but also enhance their visibility and credibility in the marketplace.
Rapid Approval primarily aims to enhance patient access to innovative treatments. By significantly reducing the approval timeline, drugs targeting serious conditions can reach patients much more quickly—an essential factor for those confronting life-threatening diseases with limited treatment options.
For instance, Clarity Pharmaceuticals' Cu-SAR-bisPSMA, which recently received expedited status, exemplifies this process. The fast track designation FDA facilitates more frequent communication with the FDA and allows for the submission of completed sections of applications as they become ready, potentially shortening review times and improving patient outcomes.
Consequently, patients can benefit from enhanced diagnosis and treatment planning, particularly in critical areas like prostate cancer, where timely access to effective therapies can prove life-saving.
Ongoing clinical programs and trials, such as the registrational Phase III trial CLARIFY—building on favorable safety and efficacy results from the PROPELLER study—further underscore the importance of expedited classification in enabling quicker access to innovative treatments, ultimately leading to better health outcomes for patients.
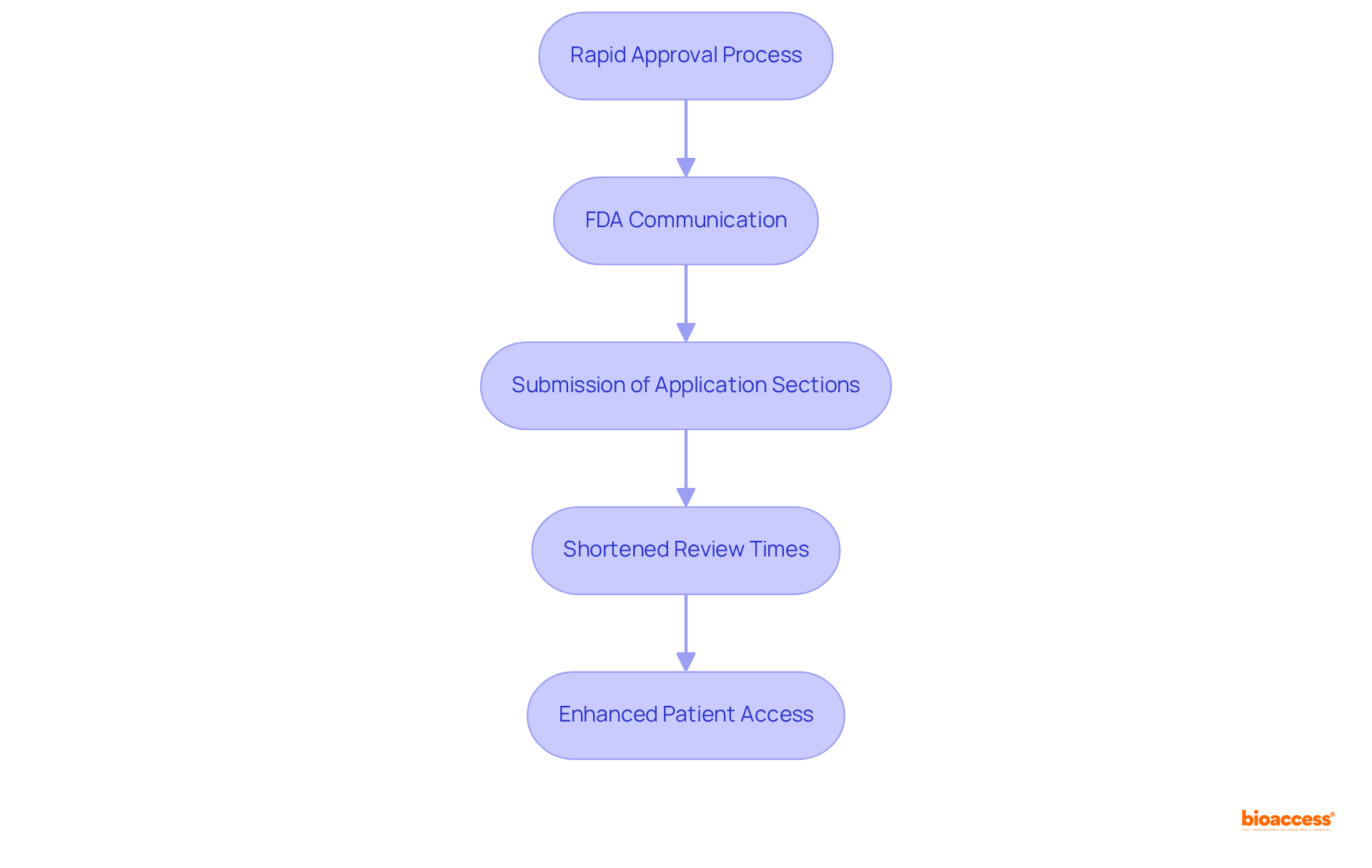
Accelerated classification significantly enhances collaboration opportunities among stakeholders, including researchers, regulatory agencies, and industry associates. This collaborative environment fosters innovation and facilitates the sharing of resources and knowledge, resulting in more efficient development processes.
For instance, medications granted Accelerated Status have been shown to reduce clinical trial durations and time to market, with a median time to initial approval that is 15 months faster than the industry average. By leveraging these partnerships, stakeholders can effectively tackle challenges and expedite project timelines.
The 2025 updates highlight that ongoing collaborations are essential, as they enable real-time adjustments and enhancements in drug development strategies. Notably, collaborations established through Accelerated Approval have demonstrated a significant impact on efficiency, with numerous firms reporting improved capabilities in managing regulatory challenges and refining trial frameworks.
At bioaccess®, our team, featuring experts like Katherine, who possesses extensive experience in medical device regulation, and Sergio, who specializes in medical innovation and artificial intelligence, plays a crucial role in navigating these processes. Their expertise directly contributes to managing the complexities of expedited approval, ensuring that projects adhere to regulatory standards and are positioned for success.
This synergy not only benefits individual projects but also advances the overarching goal of delivering innovative therapies to patients more swiftly.
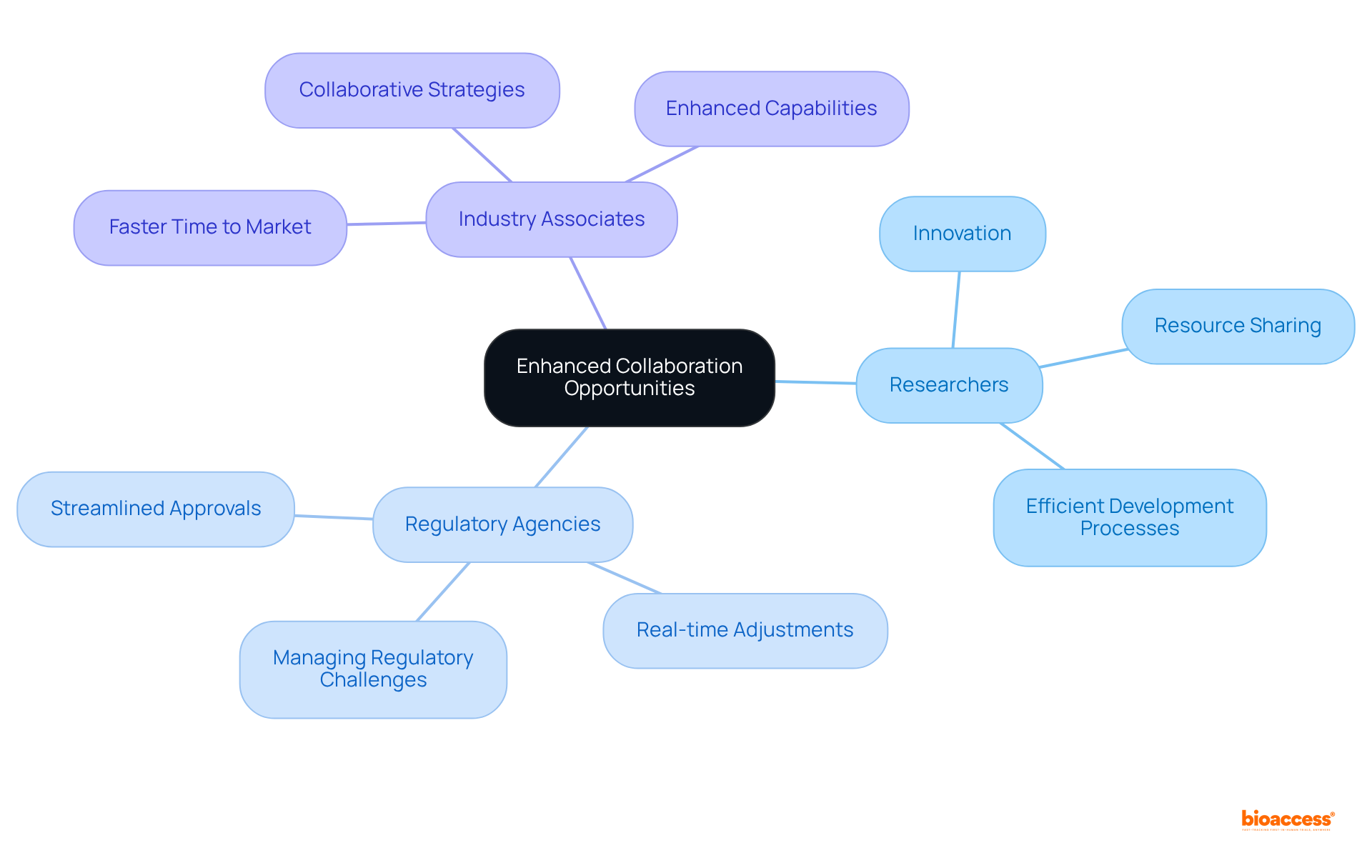
The fast track designation FDA significantly alleviates the regulatory burden faced by pharmaceutical developers by facilitating more flexible submission processes and enhancing communication with the FDA. This streamlined methodology empowers companies to navigate the approval landscape with heightened efficiency, ultimately conserving valuable time and diminishing compliance complexities. Consequently, developers can direct their focus toward innovation instead of being encumbered by regulatory obstacles.
For instance, the fast track designation FDA has allowed companies such as X4 Pharmaceuticals to engage in more frequent dialogues with the FDA, thereby expediting their clinical trials and increasing their chances of swift approval.
Furthermore, the FDA's dedication to refining these pathways under the 21st Century Cures Act has resulted in improved patient outcomes, as evidenced by the ongoing studies for therapies like Sanofi's SAR446597, which seeks to address significant unmet medical needs. This proactive regulatory environment not only nurtures innovation but also ensures that potentially life-saving treatments are delivered to patients with greater urgency.
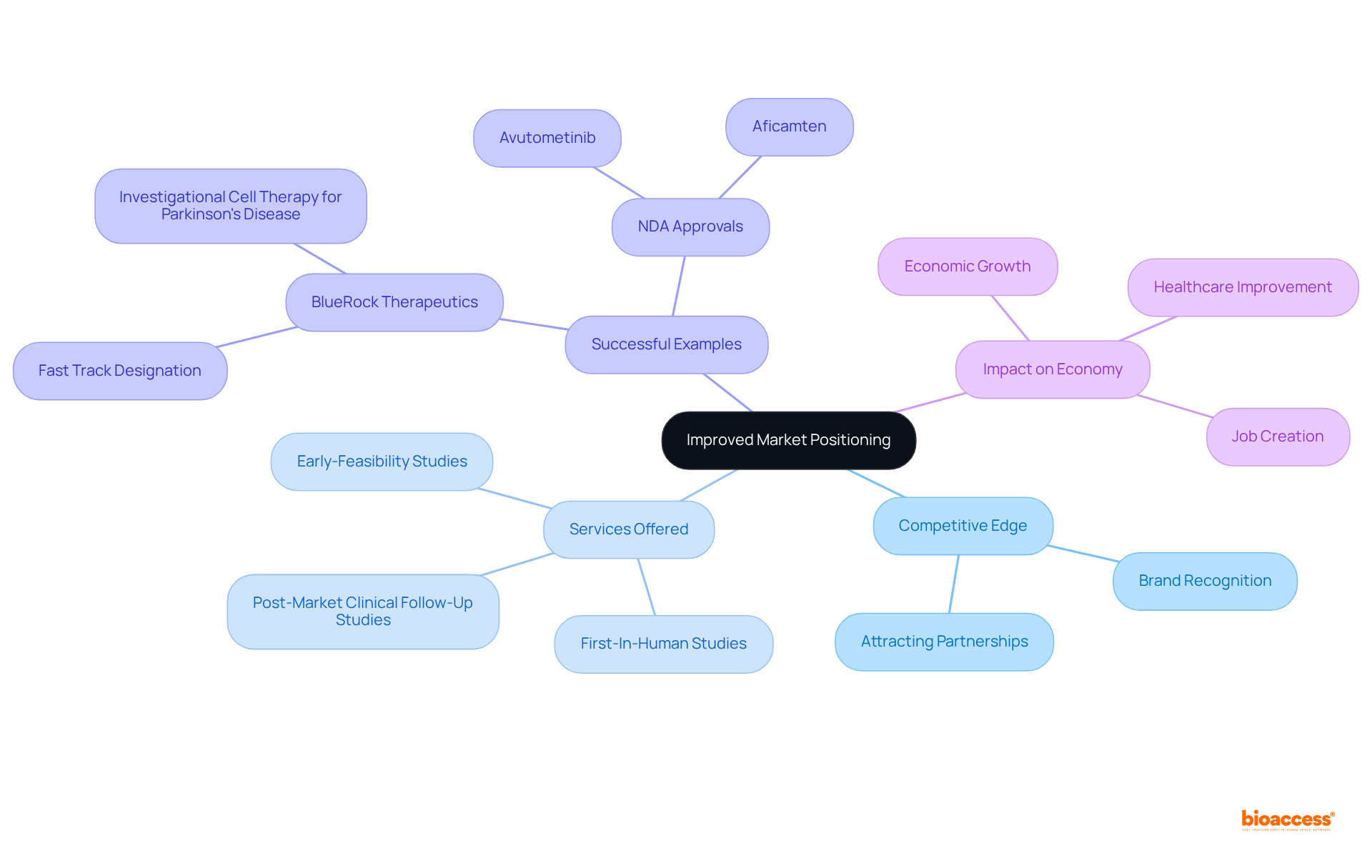
Obtaining expedited status offers businesses a substantial advantage in market positioning. By being among the first to introduce innovative therapies, these organizations can establish themselves as frontrunners in their respective fields. This competitive edge not only enhances brand recognition but also attracts potential partnerships and investment opportunities, thereby reinforcing their market presence.
For example, bioaccess® provides comprehensive clinical trial management services, including:
These services are crucial for navigating the complexities of medical device trials. Such expertise significantly improves a company's ability to attain fast track designation FDA, as demonstrated by BlueRock Therapeutics, which received this designation for its investigational cell therapy and has positioned itself as a leader in advancing treatments for Parkinson's disease.
Furthermore, the approval of NDAs for medications such as avutometinib and aficamten illustrates how the fast track designation FDA can facilitate prompt market access, a critical factor for success in the pharmaceutical sector. The impact of Medtech clinical studies extends beyond individual companies, contributing to local economies through job creation, economic growth, and healthcare improvement, making these strategic advantages essential in an industry where timely market access can dictate success.
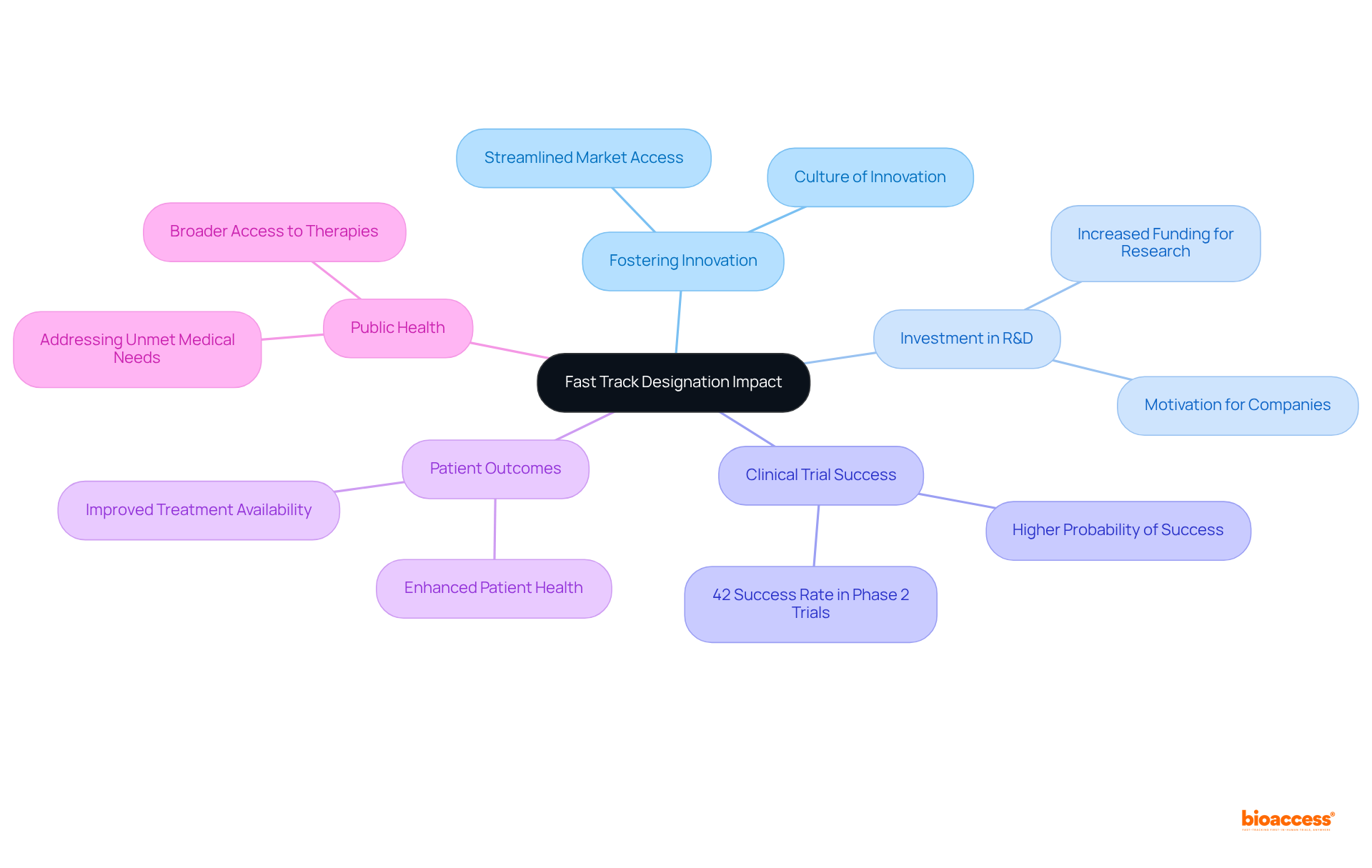
Accelerated classification plays a pivotal role in fostering innovation within the pharmaceutical industry. By streamlining market access for transformative therapies, this designation motivates companies to amplify their investments in research and development. The expedited approval process not only hastens the availability of groundbreaking treatments but also cultivates a culture of innovation that benefits the entire medical community.
Notably, therapies that have achieved expedited status demonstrate significant advancements in tackling severe conditions, underscoring the designation's impact on promoting research efforts. Statistics indicate that drugs awarded this designation enjoy a higher probability of success in clinical trials, boasting an estimated 42% success rate in Phase 2 trials, in contrast to the industry average.
This acceleration not only expedites the transition from laboratory to market but also improves patient outcomes and public health, thereby reinforcing the critical role of fast track designation FDA in the dynamic landscape of pharmaceutical innovation.
The fast track designation from the FDA stands as a pivotal mechanism in the drug development landscape, offering significant advantages that streamline the journey from innovation to market. By enhancing communication with regulatory bodies, expediting review processes, and fostering collaboration, this designation not only accelerates the approval timeline but also bolsters the financial viability of pharmaceutical projects. Ultimately, it positions companies to bring vital therapies to patients in need much sooner.
Key insights throughout the discussion highlight the multifaceted benefits of fast track designation:
In reflection, the significance of fast track designation extends beyond individual companies; it plays a crucial role in driving innovation within the pharmaceutical industry. By reducing regulatory burdens and promoting collaboration among stakeholders, this designation fosters an environment where transformative therapies can thrive. As the pharmaceutical landscape continues to evolve, leveraging the benefits of fast track designation will be essential for companies aiming to meet the urgent needs of patients and advance public health. Embracing this pathway can lead to a brighter future for both innovators and those they serve.
What is bioaccess® and what services does it provide?
bioaccess® specializes in simplifying the Rapid Pathway process for pharmaceutical development, offering over 20 years of experience in regulatory landscapes across Latin America, the Balkans, and Australia. It helps Medtech, Biopharma, and Radiopharma innovators secure ethical approvals within 4-6 weeks and provides rapid site activation and patient recruitment.
How does bioaccess® improve the drug development timeline?
bioaccess® accelerates the drug development timeline by guaranteeing rapid site activation and achieving a 50% faster enrollment rate compared to traditional markets. This strategic approach aids companies in obtaining fast track designation from the FDA for their therapies.
What is the significance of the fast track designation from the FDA?
The fast track designation from the FDA enhances communication between medication developers and the FDA, allowing for more frequent interactions and early addressing of questions. This can lead to a median time reduction of 15 months in the approval process and facilitates earlier access to potentially life-saving treatments for patients.
What are the benefits of the Rapid Pathway title in medication evaluation?
The Rapid Pathway title significantly accelerates the evaluation process for medications targeting severe conditions by allowing rolling reviews. This means portions of the application can be submitted and reviewed as they are completed, enabling quicker FDA feedback and adjustments, thus enhancing the likelihood of successful outcomes.
What types of studies does bioaccess® manage for medical devices?
bioaccess® manages various studies for medical devices, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF). This expertise helps navigate regulatory pathways effectively.
How can companies expedite their clinical trials with bioaccess®?
Companies can expedite their clinical trials by leveraging bioaccess®'s expertise in regulatory approval, clinical research site activation, and patient recruitment, which can start trials 40% faster and navigate regulatory complexities with confidence.