


The landscape of medical device regulation is continuously evolving, with Periodic Safety Update Reports (PSURs) emerging as a critical component for compliance and safety monitoring. As manufacturers navigate the complexities of regulatory requirements, understanding the essential elements of PSURs becomes paramount for ensuring product safety and maintaining market access. Yet, with increasing scrutiny and evolving standards, how can manufacturers effectively prepare for the challenges that lie ahead?
This article delves into eight vital insights that can empower medical device producers to not only meet compliance expectations but also enhance their operational efficiency and product oversight. By grasping these insights, manufacturers can position themselves to thrive in a competitive environment, ensuring they remain compliant while also fostering innovation and safety in their product offerings.
bioaccess® excels in streamlining the compliance process for medical device producers, leveraging its extensive understanding of regulatory frameworks across Latin America, the Balkans, and Australia. By emphasizing ethical approvals and rapid enrollment, bioaccess® enables producers to effectively meet regulatory requirements, significantly shortening time to market while enhancing product security oversight through FDA/EMA/MDR-ready datasets and centralized monitoring. Their adept navigation of diverse regulatory landscapes empowers clients to maintain compliance, allowing them to focus on innovation and development.
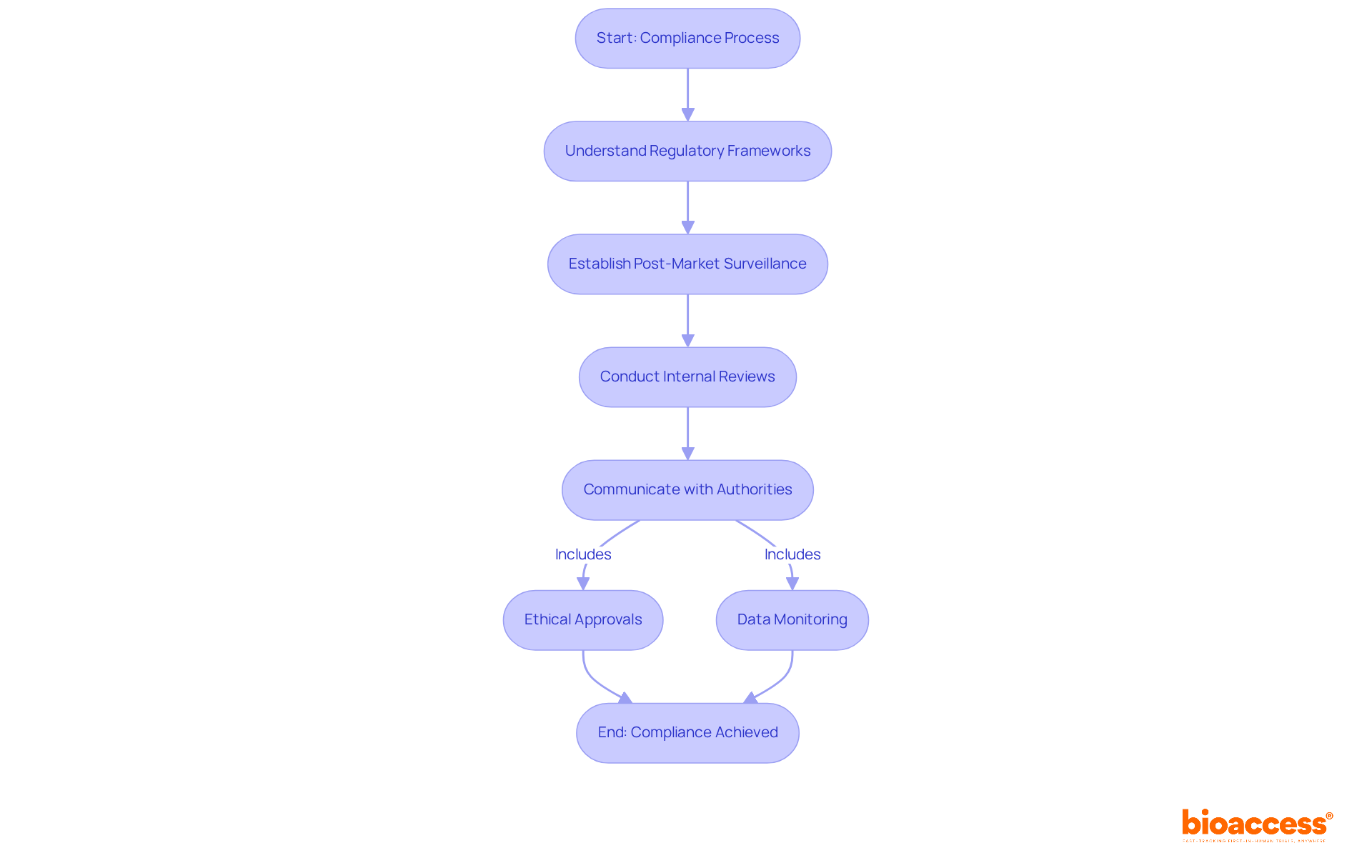
Recent developments in PSUR compliance underscore the necessity for producers to adopt proactive strategies for the psur medical device. Establishing robust post-market surveillance programs for the psur medical device and conducting regular internal reviews are essential steps. Regulatory experts stress that effective communication with authorities, including ANVISA, is crucial for fostering trust and ensuring adherence to safety regulations. As the regulatory environment evolves, particularly in Latin America and Australia, bioaccess® remains a vital partner for manufacturers aiming to navigate these complexities and achieve compliance success.
Furthermore, with ANVISA's new requirement starting in 2025 for a statement from plant suppliers affirming compliance with Good Agricultural and Collection Practices, bioaccess®'s comprehensive post-market surveillance services become even more essential. The legal obligations of the periodic safety update report under the Commission implementing Regulation (EU) No 520/2012 further emphasize the significance of complying with these changing standards.
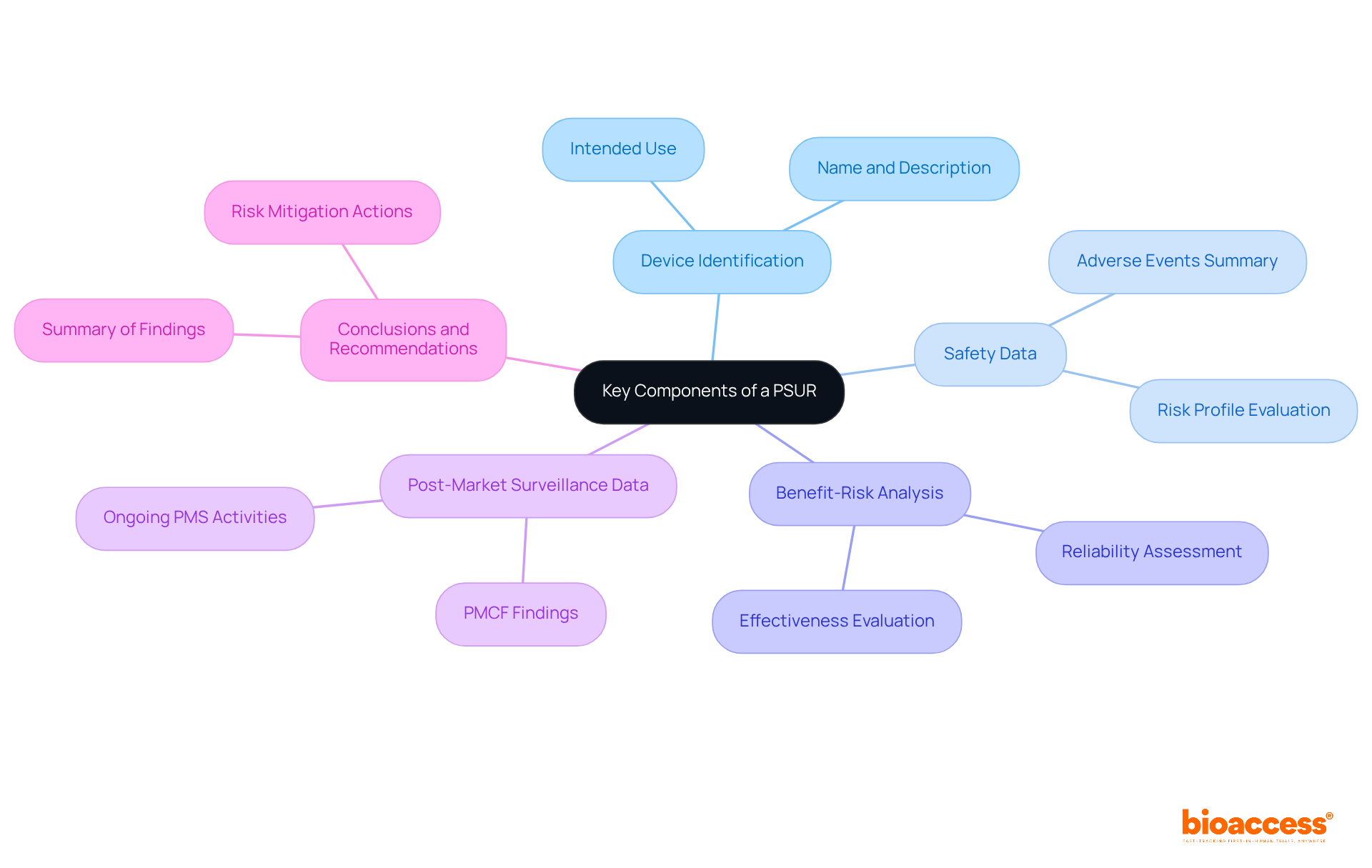
A PSUR medical device is crucial for ensuring compliance and safety monitoring under EU MDR regulations. This report must encompass several essential components:
These components together provide a thorough overview of the apparatus's safety profile, aiding informed decision-making and adherence to regulatory standards. Recent updates indicate that producers must submit PSURs annually for Class III and IIb products, while Class IIa items require updates every two years. It is essential for producers to demonstrate compliance with Article 83 of the MDR through a detailed PMS plan. Furthermore, the periodic safety update report is vital for ensuring the reliability and effectiveness of the PSUR medical device throughout its entire lifecycle. According to the Medical Device Coordination Group (MDCG) guidance from December 2022, manufacturers can find assistance in comprehending periodic safety update report requirements. Statistics indicate that a considerable proportion of medical instruments in the EU comply with post-marketing surveillance requirements, emphasizing the necessity of routine data examination and documentation in ensuring product safety and effectiveness.
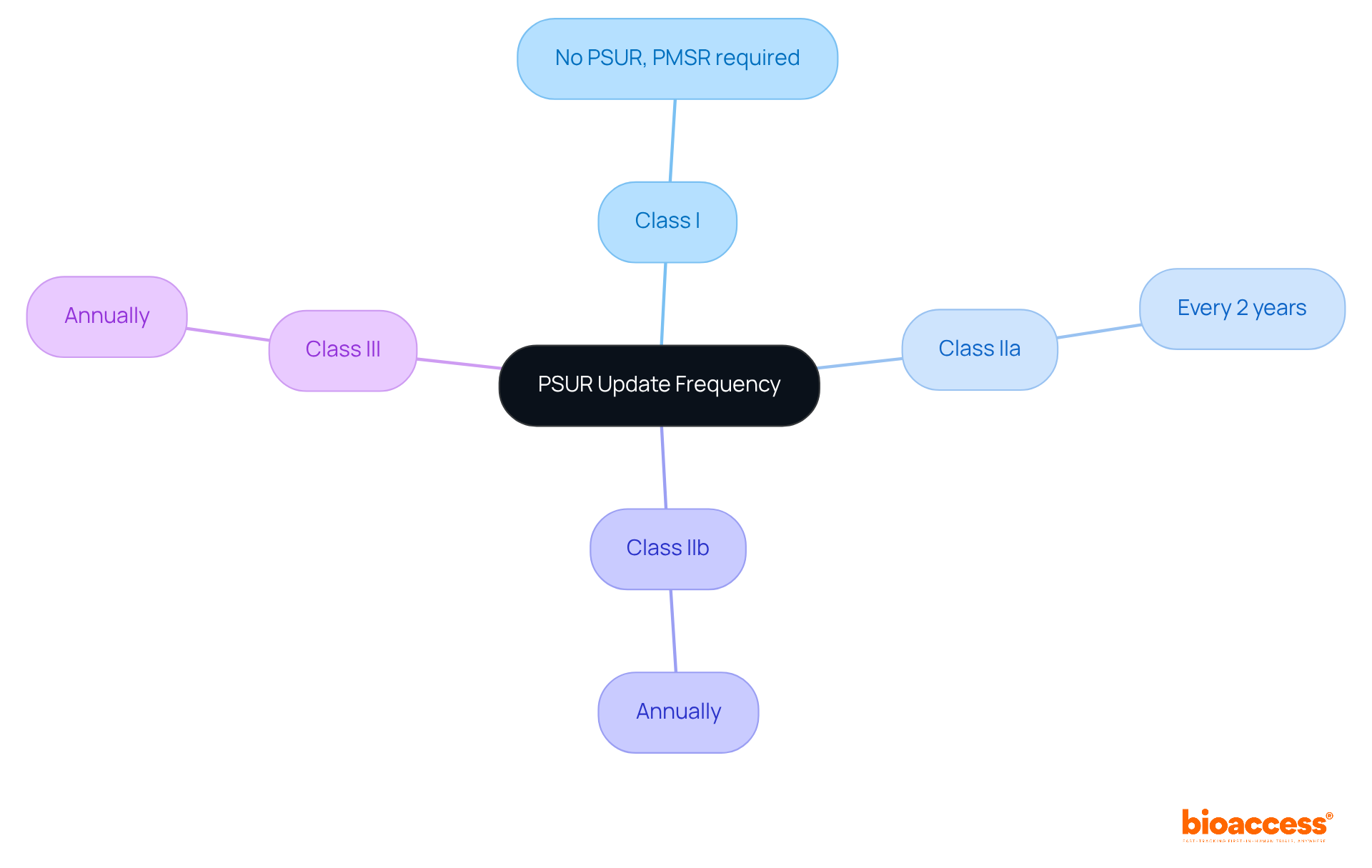
Understanding the frequency of PSUR medical device updates is crucial for producers in the Medtech landscape, particularly under the EU MDR regulations.
These timelines are not just regulatory requirements; they are essential for ensuring timely submissions and compliance with expectations. By grasping these requirements, producers can navigate the complexities of clinical research more effectively.
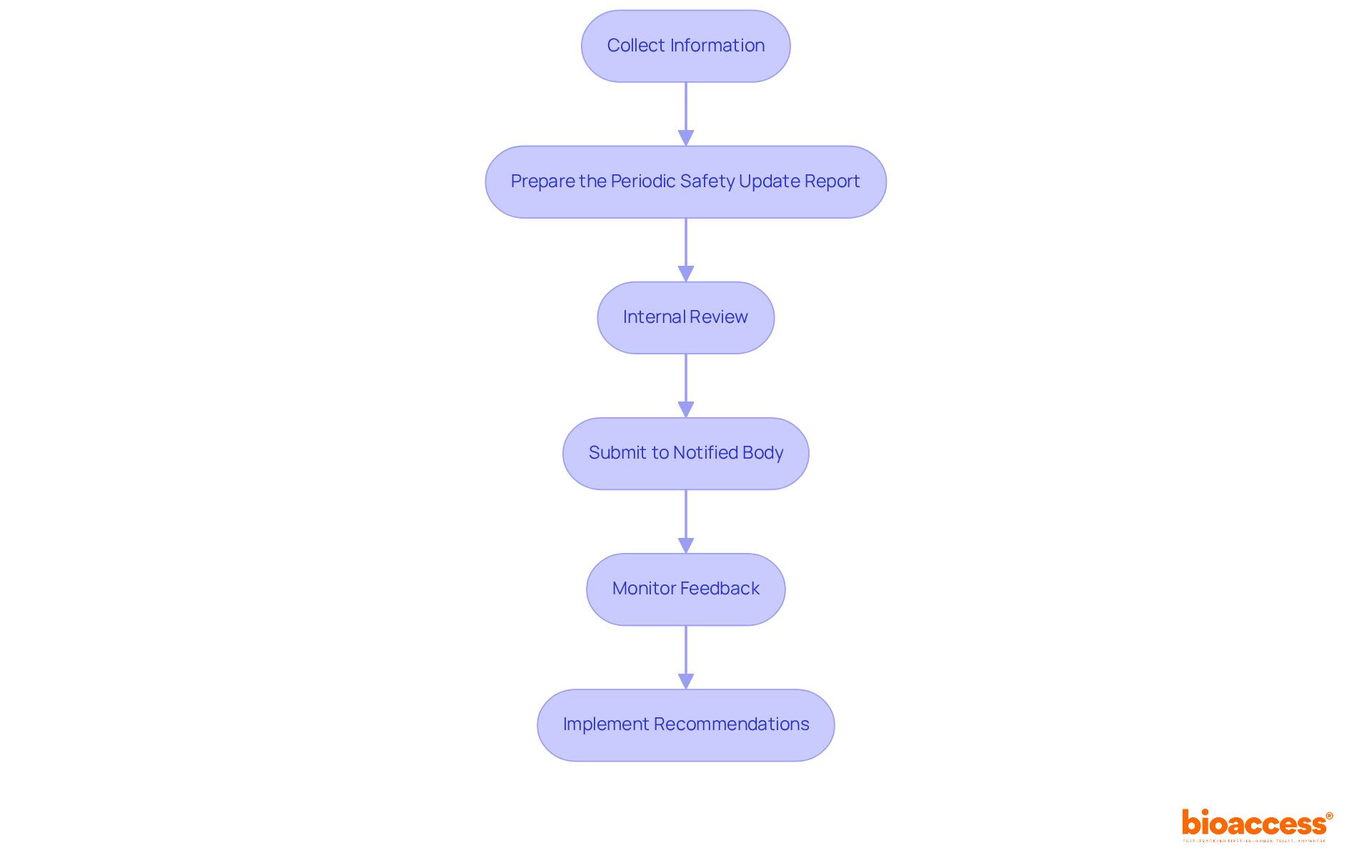
To navigate the PSUR submission process effectively, manufacturers must follow these essential steps:
Collect Information: Assemble all pertinent performance and risk information from post-market surveillance (PMS) activities. This ensures thorough coverage of incidents and user feedback, which is critical for informed decision-making.
Prepare the Periodic Safety Update Report: Incorporate all necessary elements according to EU MDR regulations. This includes an executive summary, safety evaluation, and benefit-risk assessment, providing a comprehensive overview of the product's performance.
Internal Review: Conduct a thorough evaluation of the periodic safety update report for accuracy and completeness. Involve cross-functional teams to ensure all perspectives are considered, guaranteeing that the document meets regulatory standards.
Submit to Notified Body: Send the periodic safety update report to the relevant regulatory authority or notified body. Ensure submission within the required timelines based on product classification. For instance, Class IIa devices necessitate a submission at least once every two years, while Class IIb and III devices must submit annually.
Monitor Feedback: Be prepared to respond to any queries or requests for additional information from regulators. Maintaining open lines of communication is vital for facilitating a smooth review process.
Implement Recommendations: Act on any suggestions or necessary actions identified in the feedback. This demonstrates a commitment to ongoing enhancement and adherence to standards.
As Sara Adams, an expert in quality systems, emphasizes, "Manufacturers must remain proactive and diligent in their compliance and risk management processes to adhere to the EU MDR guidelines."
By following these steps, manufacturers can enhance their PSUR medical device submission process, effectively addressing common challenges and ensuring adherence to regulatory requirements.
Post-Market Surveillance (PMS) is essential for creating Periodic Safety Update Reports (PSURs), which provide ongoing insights into the safety and performance of medical devices after they hit the market. Key PMS activities include:
These activities are fundamental for developing a comprehensive PSUR that accurately reflects the product's risk profile. As Brittani Smith, a medical equipment expert, emphasizes, "You should be focusing on these processes during design and development, so that when your product reaches the market, you’re ready to collect post-market data and respond to it promptly." Effective PMS not only enhances the accuracy of the PSUR but also demonstrates a producer's commitment to patient well-being and regulatory compliance. By leveraging PMS data, producers can ensure that their PSURs are informed by real-world evidence, ultimately leading to better decision-making and improved product safety.
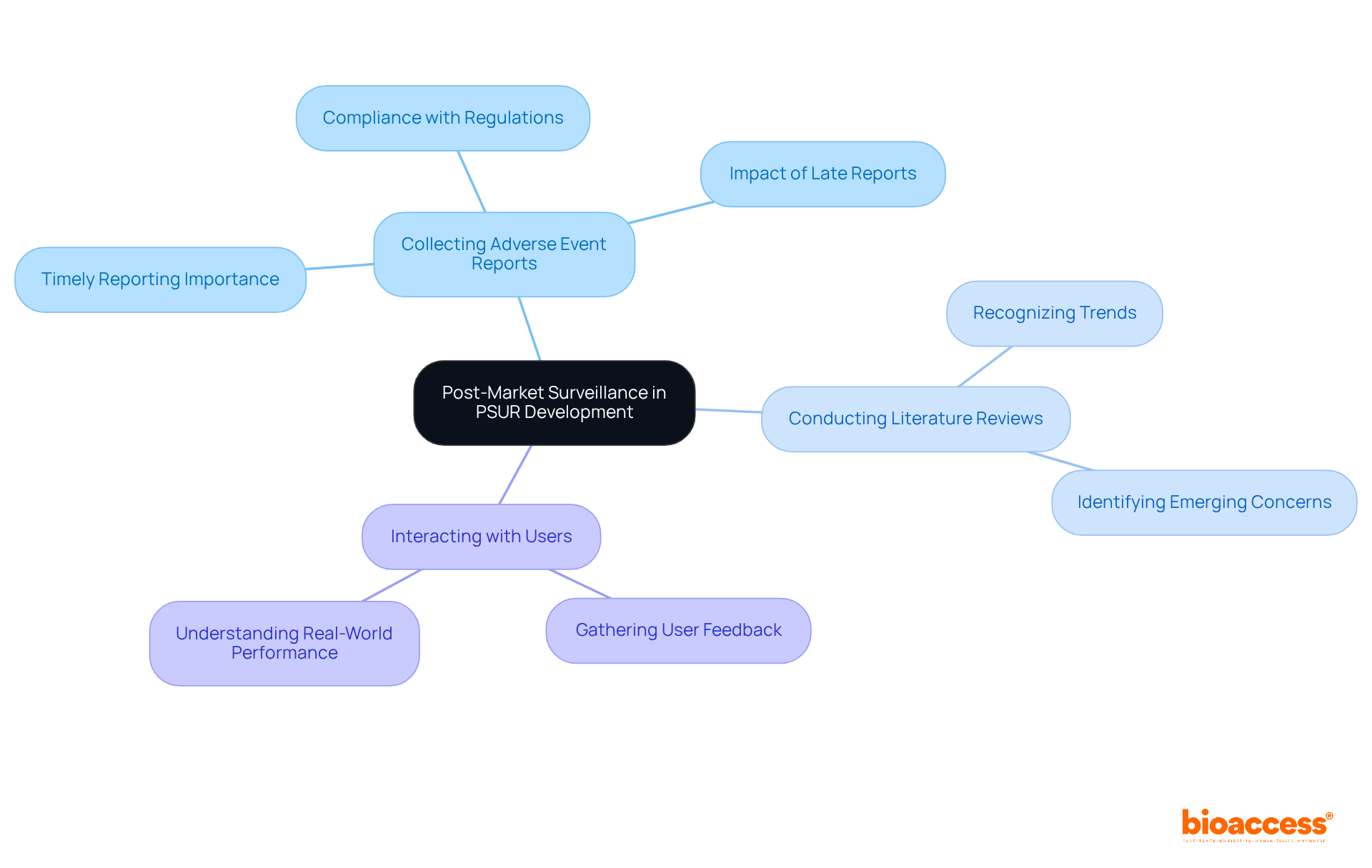
Manufacturers must navigate several critical regulatory requirements when preparing PSUR medical device reports.
Compliance with EU MDR requires that PSUR medical devices must adhere to the guidelines established in the EU Medical Device Regulation (2017/745). This ensures that all documentation meets the necessary standards for safety and efficacy, which is paramount in the Medtech landscape.
Content requirements for each PSUR medical device must encompass specific components, including thorough risk information, benefit-risk assessments, and findings from post-market clinical follow-up (PMCF). This guarantees that the reports offer a comprehensive summary of the equipment's performance in real-world environments, fostering trust among stakeholders.
Failure to comply with these requirements can result in substantial penalties, such as limitations on market access. This emphasizes the significance of following regulatory standards for manufacturers striving to thrive in the competitive medical device environment.
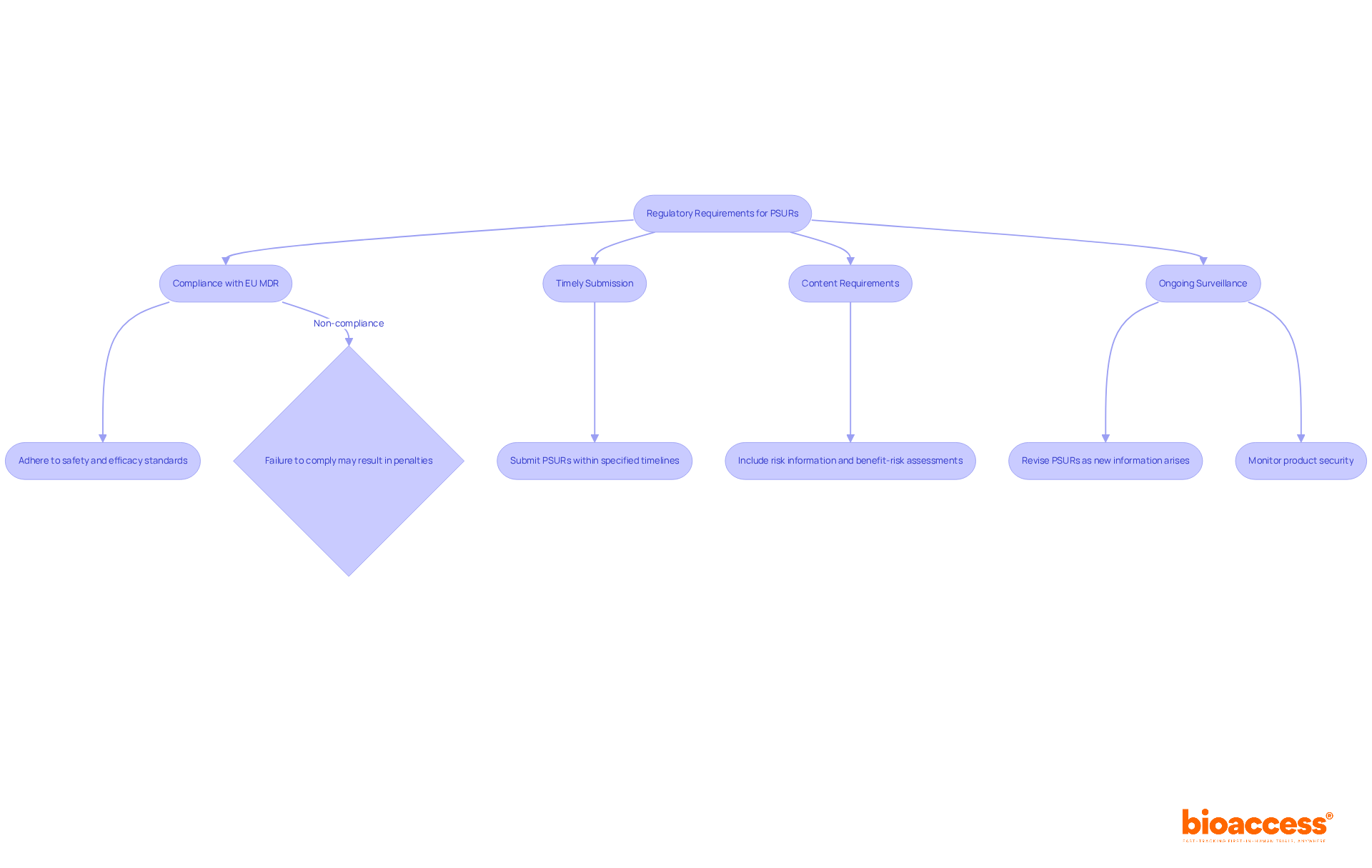
Regulators evaluate Periodic Safety Update Reports (PSURs) based on several critical criteria:
In Colombia, the National Food and Drug Surveillance Institute (INVIMA) plays a pivotal role in supervising the regulatory compliance of medical instruments, including the assessment of the psur medical device. As a Level 4 health authority recognized by the Pan American Health Organization/World Health Organization, INVIMA ensures that manufacturers adhere to stringent safety and efficacy standards. INVIMA's responsibilities include monitoring and controlling medical devices, suggesting technical standards, and ensuring compliance with health regulations.
Manufacturers should prioritize these criteria for the psur medical device to facilitate a smooth assessment process. Recent data indicates that common deficiencies in submitted PSURs often relate to incomplete data presentation and lack of clarity, which can hinder regulatory approval. For instance, the median workload for periodic safety update reports is estimated at 868 entries each year, with a possible rise of 15%. By addressing these areas, companies can enhance their compliance success and ensure that their PSURs meet regulatory expectations effectively. Consistently reviewing and updating safety report processes based on the latest regulatory expectations is essential for maintaining compliance.
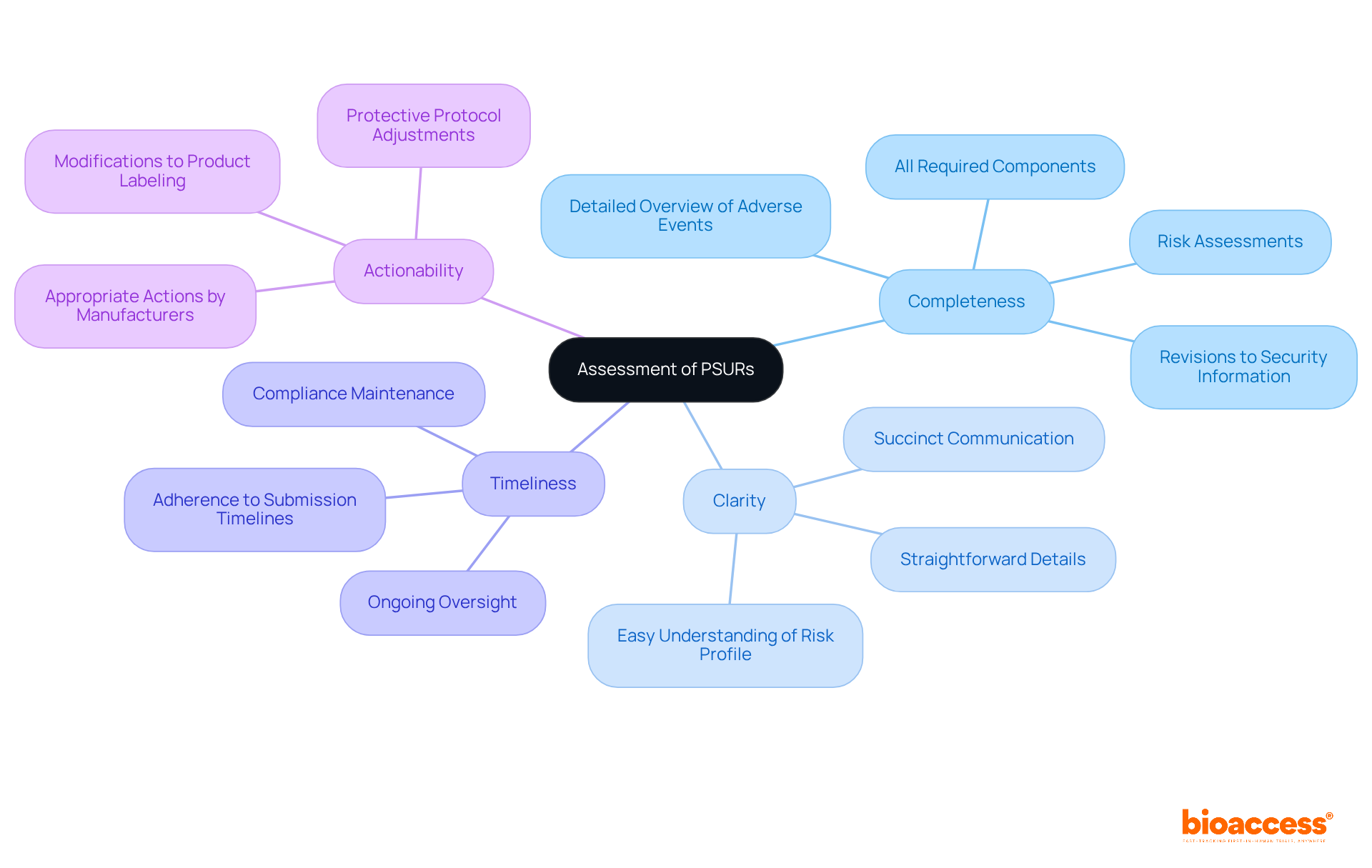
Missing PSUR submission deadlines can lead to several serious consequences for manufacturers:
To prevent these outcomes, producers should focus on prompt periodic safety update reports. By prioritizing timely submissions, manufacturers not only safeguard their operations but also enhance their credibility in the Medtech landscape.
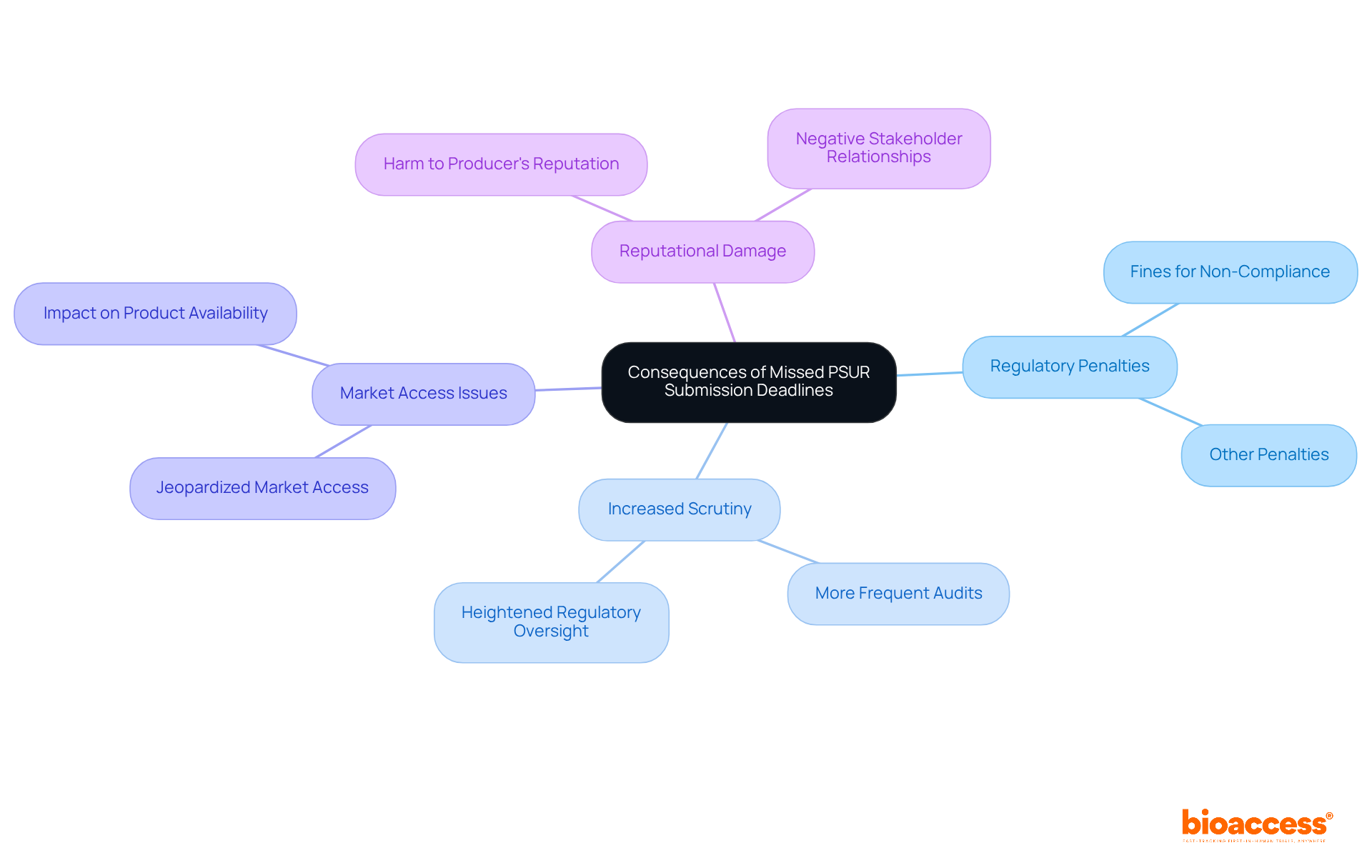
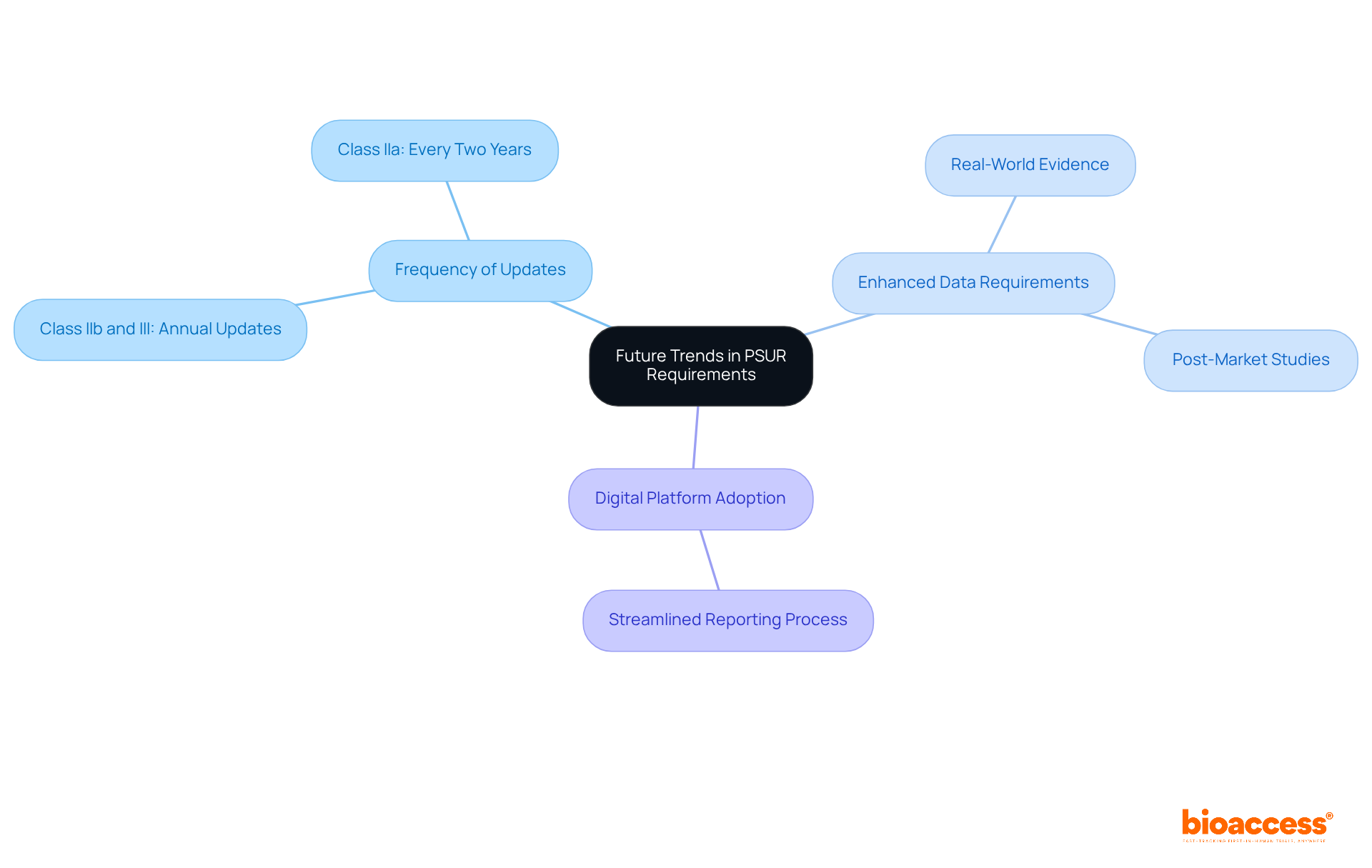
As regulatory landscapes evolve, manufacturers must prepare for emerging trends in specifications for the PSUR medical device. This preparation is crucial for ensuring compliance and maintaining the safety of the PSUR medical device in a rapidly changing environment.
Regulatory bodies are likely to mandate more frequent updates of the PSUR medical device, reflecting a heightened emphasis on safety monitoring. Recent trends indicate that Class IIb and Class III instruments now require annual updates, while Class IIa instruments must be updated every two years. This shift underscores the need for more rigorous oversight concerning the PSUR medical device.
Enhanced Data Requirements: Future PSUR medical device reports may necessitate the inclusion of more extensive data derived from real-world evidence and post-market studies. This transition aims to guarantee that producers provide a comprehensive perspective on their products' performance and security in real-world usage situations.
The adoption of digital platforms for PSUR medical device submissions and monitoring is expected to become standard practice. This transition will streamline the reporting process and enhance the efficiency of data collection and analysis, in line with the guidance provided by the Medical Device Coordination Group (MDCG) in December 2022.
Producers should remain vigilant and adjust their methods to align with these trends, ensuring continuous compliance and the safety of their medical products. With experts like Ana Criado, who has extensive experience in regulatory affairs, including her leadership role at INVIMA and her academic background in biomedical engineering, and Katherine Ruiz, specializing in regulatory affairs for medical devices and in vitro diagnostics in Colombia, the industry can navigate these changes effectively.
Understanding the complexities of Periodic Safety Update Reports (PSURs) is crucial for medical device manufacturers aiming for compliance in a rapidly evolving regulatory landscape. Effective PSUR management not only ensures adherence to EU MDR regulations but also enhances product safety and fosters trust among stakeholders. By prioritizing compliance strategies and leveraging resources like bioaccess®, manufacturers can navigate these challenges more efficiently.
Key insights regarding the essential components of a PSUR, the importance of post-market surveillance, and the specific requirements based on device classifications have been highlighted. The necessity for timely submissions and the consequences of non-compliance underscore the need for manufacturers to remain proactive in their compliance efforts. Furthermore, future trends indicate an increasing demand for rigorous oversight and enhanced data requirements in PSUR reporting.
As the regulatory environment continues to evolve, manufacturers are encouraged to stay informed and adaptable. Embracing best practices for PSUR compliance and investing in effective post-market surveillance systems can significantly improve safety outcomes and regulatory adherence. By taking these proactive steps, medical device producers not only protect their market access but also contribute to overall patient safety and industry integrity.
What is bioaccess® and what role does it play for medical device manufacturers?
bioaccess® is a service that streamlines the compliance process for medical device manufacturers by leveraging its understanding of regulatory frameworks across Latin America, the Balkans, and Australia. It helps producers meet regulatory requirements, shorten time to market, and enhance product security oversight.
What are the key components of a Periodic Safety Update Report (PSUR) under EU MDR regulations?
The key components of a PSUR include device identification, safety data, benefit-risk analysis, post-market surveillance (PMS) data, and conclusions and recommendations. These elements help ensure compliance and monitor safety.
How often do different classes of medical devices need to update their PSUR?
Class I devices do not require a PSUR; instead, they need a Post-Market Surveillance Report (PMSR). Class IIa devices require updates every two years, Class IIb devices need annual updates, and Class III devices must submit PSURs annually.
Why is post-market surveillance important for medical device manufacturers?
Post-market surveillance is essential for monitoring the safety and effectiveness of medical devices after they are on the market. It helps manufacturers comply with regulations and manage risks by providing insights from ongoing PMS activities.
What new requirement will ANVISA enforce starting in 2025?
Starting in 2025, ANVISA will require a statement from plant suppliers affirming compliance with Good Agricultural and Collection Practices, making post-market surveillance services even more critical for manufacturers.
What should manufacturers do to ensure compliance with PSUR regulations?
Manufacturers should establish robust post-market surveillance programs, conduct regular internal reviews, and maintain effective communication with regulatory authorities to ensure adherence to safety regulations and build trust.
How does bioaccess® assist clients in navigating regulatory complexities?
bioaccess® assists clients by providing expertise in regulatory frameworks, enabling them to maintain compliance and focus on innovation and development while managing the challenges posed by evolving regulations.
In 2024, the regulatory landscape for pharmacovigilance (PV) reporting has been continued to evolve rapidly.
🔍… | Shreyashi K. (https://linkedin.com/posts/shreyashikundurph_lets-discuss-about-major-regulatory-trends-activity-7244241079120621570-lzqo)