


This article examines the essential components that underpin the success of informed consent form (ICF) clinical trials. It asserts that the effectiveness of ICF clinical trials hinges on strategic factors, including:
These elements not only streamline processes but also enhance participant understanding and engagement. Ultimately, this leads to improved trial outcomes, highlighting the critical role these factors play in the clinical research landscape.
In the rapidly evolving realm of medical research, the success of informed consent form (ICF) clinical trials relies on a delicate equilibrium of ethical standards, regulatory compliance, and participant engagement. As the demand for innovative medical solutions escalates, grasping the key elements that drive effective ICF trials is crucial for researchers and sponsors alike.
What strategies can be employed not only to streamline the process but also to ensure that participants are well-informed and actively engaged throughout the trial? This article explores eight essential elements that can revolutionize ICF clinical trials, enhancing both efficiency and participant satisfaction.
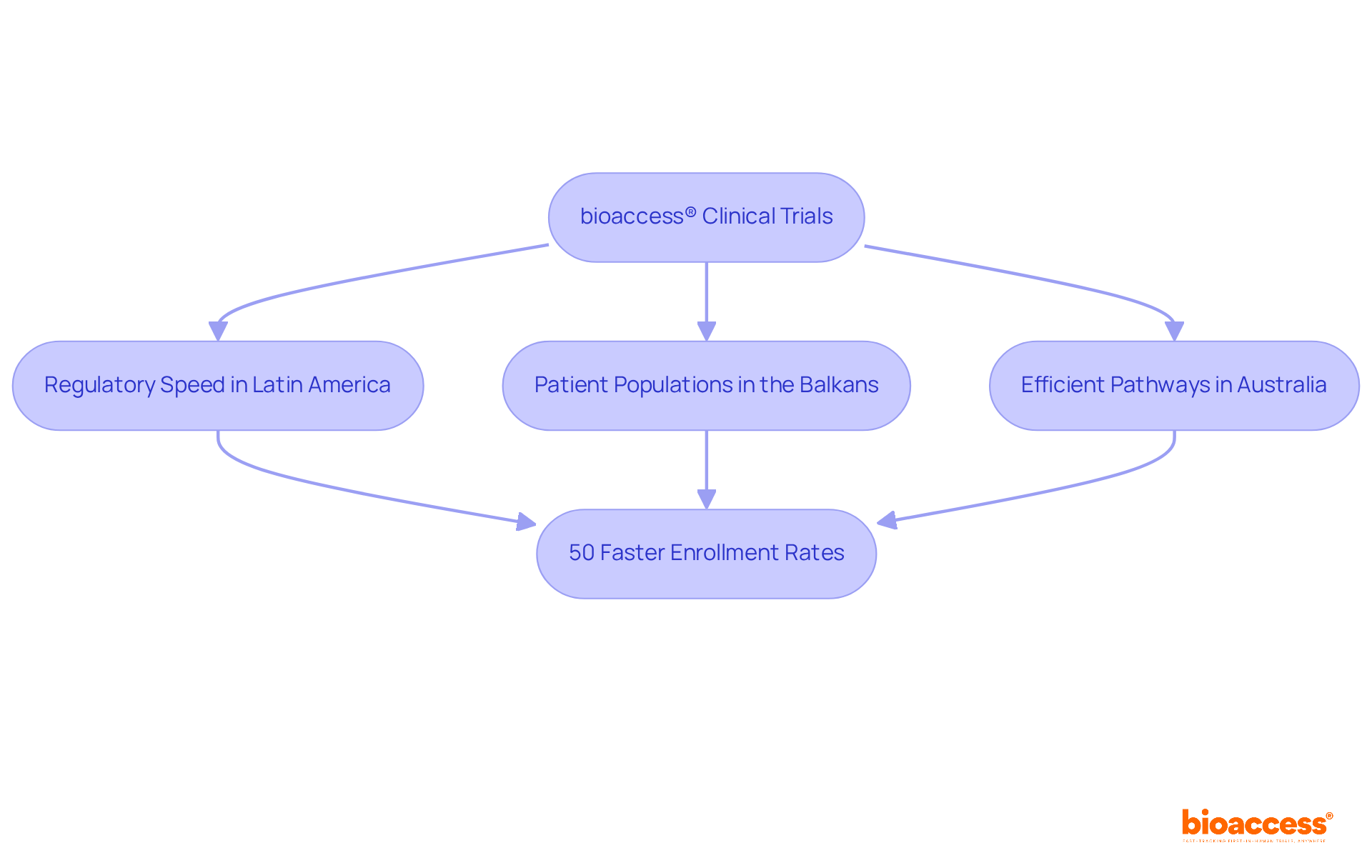
bioaccess® strategically leverages the regulatory speed of Latin America, the diverse patient populations of the Balkans, and Australia's efficient pathways to achieve ethical approvals in a remarkable timeframe of just 4 to 6 weeks. This powerful combination results in enrollment rates that are 50% faster than those found in conventional markets, thereby positioning bioaccess® as a leader in the expedited informed consent form (ICF clinical trial) studies.
By streamlining the clinical research process, bioaccess® not only facilitates rapid advancements in medical technology but also empowers innovators to bring their products to market more swiftly. This ultimately enhances patient access to cutting-edge therapies on a global scale, underscoring the importance of collaboration in addressing key challenges within the Medtech landscape.
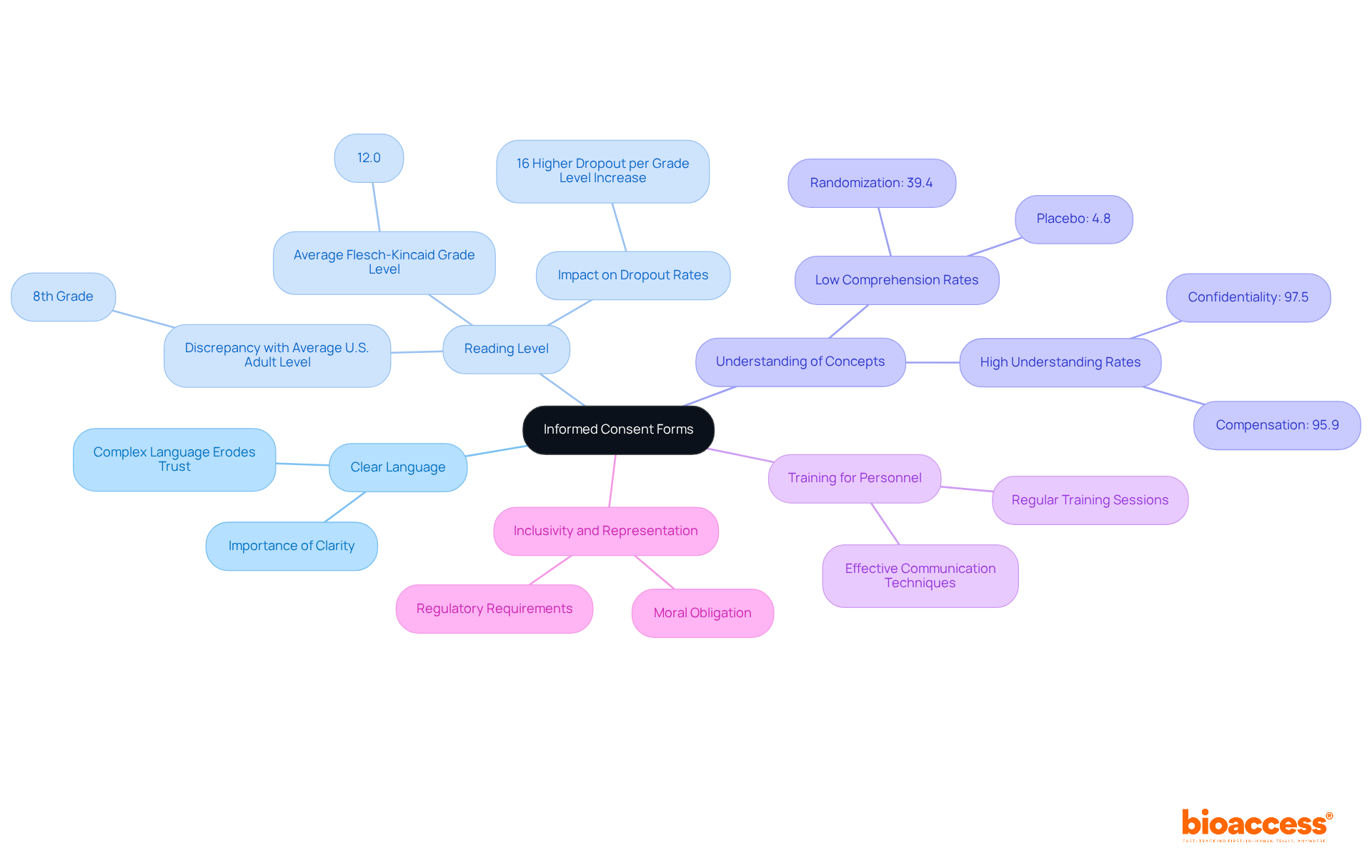
Informed Consent Forms (ICFs) are critical for ensuring that individuals fully understand the nature of the ICF clinical trial, the associated risks, and their rights. To achieve this, ICFs must be crafted in clear, accessible language, providing comprehensive details about the study's objectives, procedures, and potential side effects.
Research indicates that the typical Flesch-Kincaid Grade Level of consent forms is 12.0, significantly above the average reading level of 8th grade for most U.S. adults. This discrepancy can hinder retention and engagement. Alarmingly, understanding rates for essential components, such as randomization and placebo, are low, with only 4.8% of individuals comprehending the concept of placebo.
Regular training sessions for research personnel on effective communication techniques can greatly enhance understanding and compliance. This proactive approach not only fosters trust but also contributes to more successful outcomes in the ICF clinical trial. Participants who are well-informed are more likely to remain engaged throughout the research process.
Furthermore, streamlining consent forms to ensure they are inclusive and representative is not merely a moral obligation; it is increasingly becoming a regulatory requirement in medical research.
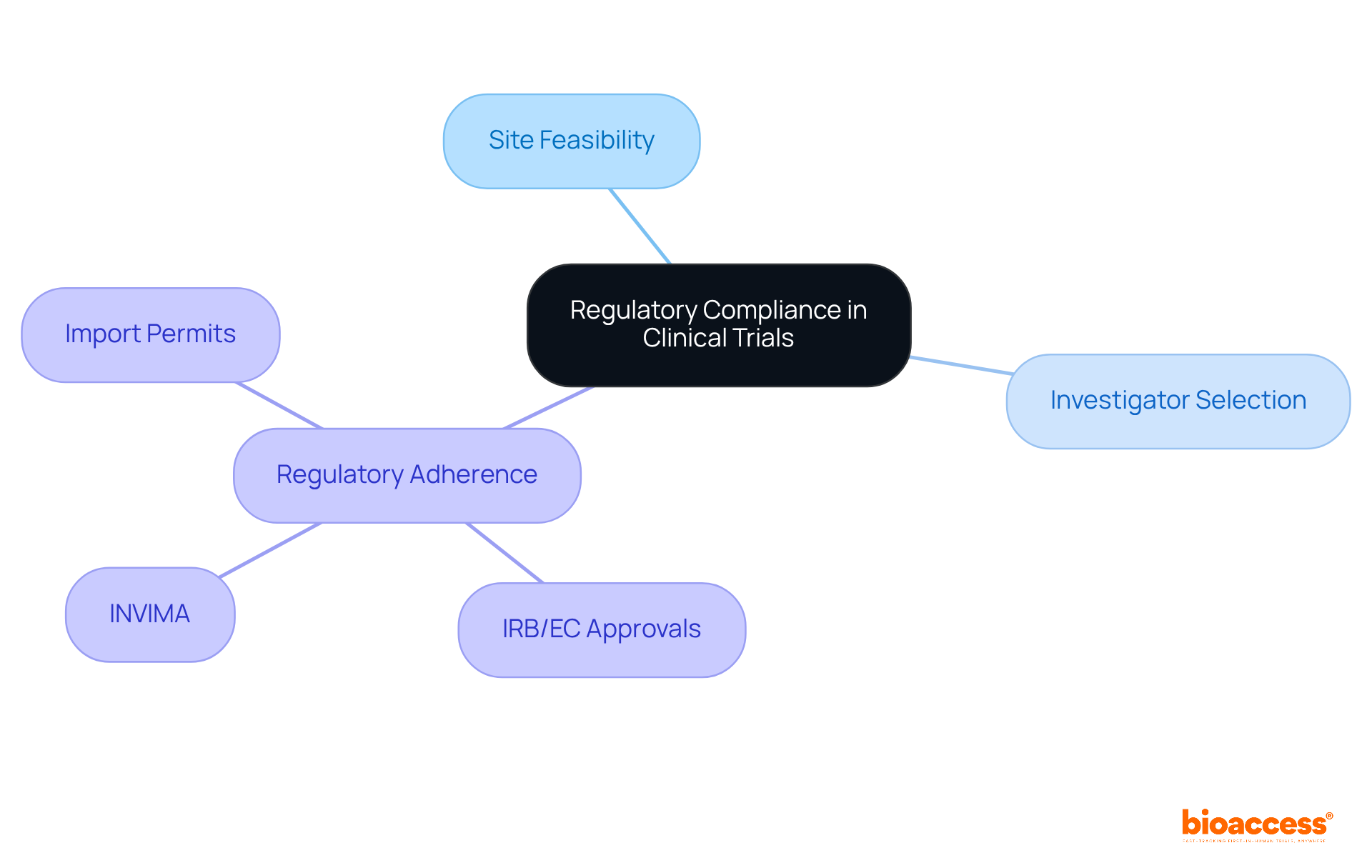
Maintaining regulatory adherence is crucial in medical studies to ensure that ethical standards are met. This necessitates compliance with the standards established by regulatory authorities, such as the FDA or EMA, while meticulously following all study protocols.
At bioaccess®, we are dedicated to supporting medical device clinical studies in Latin America. Our comprehensive approach includes:
Our expertise in securing necessary approvals, including IRB/EC and INVIMA, along with managing import permits, guarantees that all aspects of the study comply with local regulations. Regular audits and training play a vital role in sustaining compliance, thereby safeguarding participants and ensuring the integrity of the research data.
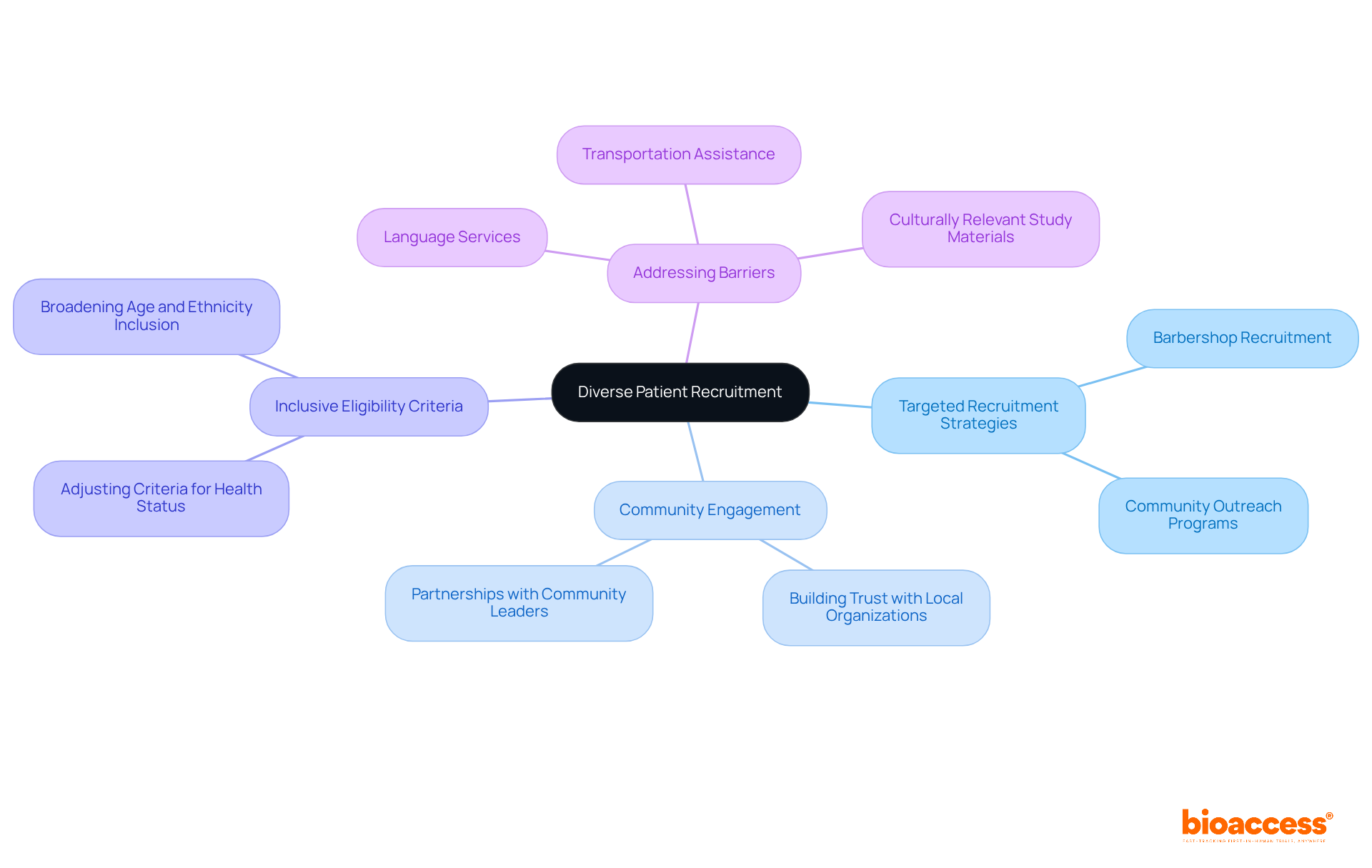
Recruiting a diverse group of patients is essential for improving the representativeness of research data. Implementing targeted recruitment strategies effectively engages various demographic groups, including different age ranges, ethnicities, and health statuses.
For instance, community-based participatory research, such as utilizing barbershops to recruit Black men, has proven successful in enhancing recruitment from underrepresented populations by fostering trust and collaboration with local organizations.
Moreover, adjusting eligibility criteria to be more inclusive creates opportunities for diverse participation, allowing researchers to capture a broader spectrum of responses to treatments. By ensuring that the research population reflects the broader community, researchers can enhance the relevance of their findings and gain valuable insights into how different populations respond to medical interventions.
This method not only encourages health equity but also enhances the overall reliability of research outcomes. Furthermore, addressing barriers faced by non-native English speakers, such as providing language interpretation services, is essential for fostering inclusivity in recruitment strategies.
Establishing robust data management systems is crucial for upholding the integrity of research data. These systems must enable precise data collection, storage, and analysis, all while ensuring compliance with regulatory requirements. At bioaccess, we provide comprehensive clinical study management services for ICF clinical trials, which include:
Routine data audits and validation checks are essential; they can reveal discrepancies early, allowing for swift corrections and ensuring the reliability of results. By integrating these services, we ensure that our data management systems are not only effective but also aligned with the highest regulatory standards.
Continuous employee education is essential for ensuring adherence to the guidelines of the ICF clinical trial in clinical studies. Comprehensive training programs must encompass:
A randomized controlled study evaluating informed consent training formats revealed that merely 46% of participants completed the training within the shortest timeframe, highlighting the challenges faced in achieving effective training compliance. By equipping research teams with the requisite knowledge and skills, organizations can significantly enhance participant interactions, thereby ensuring that all consent procedures are executed ethically and efficiently.
As John F. Kennedy aptly stated, 'Leadership and learning are indispensable to each other,' emphasizing the vital link between training and effective leadership in ICF clinical trials. This dedication to ongoing education not only cultivates a culture of compliance but also empowers staff to adeptly navigate the complexities of informed consent with confidence and integrity.
Incorporating technology into the informed consent procedure significantly streamlines operations and enhances engagement among participants. Electronic consent (eConsent) platforms provide engaging, user-friendly experiences, ensuring individuals fully comprehend study details. These platforms facilitate real-time data collection and monitoring, improving overall trial efficiency and bolstering compliance with regulatory standards.
By automating consent tracking and minimizing manual errors, eConsent solutions can reduce consent-related protocol deviations by up to 57%, leading to more reliable study outcomes. Furthermore, eConsent enhances participant retention and engagement, achieving retention rates that are 30% higher compared to traditional paper-based methods.
As the adoption of eConsent continues to rise, it is poised to become a lasting component in research, offering significant advantages to all stakeholders involved.
Regular monitoring and auditing are vital components in ensuring protocol adherence within ICF clinical trials. This systematic approach encompasses comprehensive evaluations of experimental activities, stringent data integrity checks, and meticulous adherence assessments. By establishing a robust monitoring system, organizations like bioaccess can identify areas for improvement and ensure that the ICF clinical trial studies are conducted in accordance with established guidelines.
For instance, a collaboration between a sponsor and a Contract Research Organization (CRO) successfully implemented centralized alerts across 22 global sites, leading to a 50% reduction in protocol deviations. Furthermore, the use of wearable pill caps in a neurology study resulted in a 40% increase in medication adherence, underscoring the impact of monitoring on subject compliance.
Addressing barriers such as complex visit schedules and insufficient communication from site staff is essential for sustaining adherence. Additionally, bioaccess's comprehensive research management services—including feasibility studies, site selection, compliance assessments, and project oversight—facilitate the implementation of clear onboarding and educational strategies.
Initiatives such as welcome kits and visual timelines can significantly enhance participant understanding and engagement. These efforts not only improve the quality and reliability of research outcomes but also foster a culture of continuous improvement, ultimately leading to more successful ICF clinical trials.
Clear communication among stakeholders is essential for the success of clinical studies. Regular updates and open lines of communication with investigators, sponsors, regulatory bodies, and ethics committees foster collaboration and build trust. Involving all parties ensures that they remain informed and engaged, resulting in more efficient operations and swift resolution of any issues. This proactive strategy not only enhances the overall experience but also contributes to achieving higher success rates by aligning expectations and enabling effective decision-making.
For instance, organizations like bioaccess that implement organized communication strategies—such as regular stakeholder meetings and clear reporting protocols—often report improved participant recruitment and retention, alongside greater adherence to regulatory requirements.
Furthermore, bioaccess's comprehensive management services for research studies, including feasibility assessments, site selection, compliance evaluations, setup, and project oversight, underscore the importance of transparency in research. Such transparency bolsters public perception and trust in study sponsors and the pharmaceutical sector, reinforcing the ethical commitment to openness as a regulatory responsibility.
Ultimately, promoting transparency in medical research transcends regulatory necessity; it embodies a commitment to ethical principles that can profoundly influence study outcomes.
Post-trial follow-up is an essential component of the clinical trial process, enabling researchers to gather critical feedback from subjects and evaluate the long-term effects of interventions. This stage not only ensures compliance with ethical standards and regulatory requirements but also fosters a sense of community and trust among contributors. Engaging with individuals after the trial significantly enhances their willingness to participate in future studies, thereby supporting the advancement of medical knowledge.
Research indicates that effective follow-up strategies, such as personalized communication and retention efforts, lead to higher response rates and increased satisfaction among participants. Additionally, collecting feedback allows researchers to identify areas for improvement in study design and execution, ultimately resulting in more reliable and ethically sound medical research. Insights from contributors can also inform the development of tailored interventions that address the unique needs of diverse populations, including marginalized groups.
By prioritizing feedback from participants, research studies can evolve to better meet the needs of both researchers and contributors, ensuring equitable sharing of research benefits. As ethical discussions underscore, the rights, safety, and well-being of study participants must extend beyond the research duration, highlighting the importance of post-study access to treatment.
Furthermore, bioaccess® emphasizes comprehensive trial management services, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), and Post-Market Follow-Up Studies (PMCF), ensuring that participant involvement and feedback are integral to the trial process. This commitment to ongoing compliance and the welfare of participants is vital for building trust and enhancing the overall success of clinical research.
A notable example of this is the collaboration with Welwaze Medical Inc. for the Celbrea® medical device launch, which underscores the importance of regulatory access and participant engagement in achieving successful outcomes.
The success of informed consent form (ICF) clinical trials is fundamentally rooted in several key elements that collectively enhance efficiency, uphold ethical standards, and boost participant engagement throughout the research process. By emphasizing strategic approaches such as:
Organizations can significantly accelerate the development and implementation of clinical trials. This ultimately leads to improved patient access to innovative therapies.
Throughout this discussion, the importance of:
has been underscored. Effectively training staff, employing robust data management systems, and fostering open communication among stakeholders are critical to achieving compliance and enhancing the overall quality of research. Moreover, the role of post-trial follow-up in gathering feedback and ensuring ongoing adherence to ethical standards is vital, as it contributes to building trust and improving future studies.
In conclusion, the landscape of ICF clinical trials is evolving, and the adoption of best practices is essential for success. Organizations are strongly encouraged to prioritize these elements to not only meet regulatory requirements but also to enhance the integrity and reliability of research outcomes. By embracing these strategies, stakeholders can collaborate effectively to foster a culture of transparency and continuous improvement in clinical research, ultimately benefiting participants and advancing medical knowledge.
What is bioaccess® and what role does it play in clinical trials?
bioaccess® accelerates informed consent form (ICF) clinical trials by leveraging the regulatory speed of Latin America, diverse patient populations in the Balkans, and efficient pathways in Australia, achieving ethical approvals in just 4 to 6 weeks.
How does bioaccess® improve enrollment rates in clinical trials?
By streamlining the clinical research process, bioaccess® achieves enrollment rates that are 50% faster than conventional markets, positioning itself as a leader in expedited ICF clinical trial studies.
Why are Informed Consent Forms (ICFs) important in clinical trials?
ICFs ensure that participants fully understand the nature of the trial, associated risks, and their rights. They must be written in clear, accessible language to facilitate comprehension and engagement.
What challenges exist regarding the readability of Informed Consent Forms?
The typical Flesch-Kincaid Grade Level of consent forms is 12.0, which is significantly higher than the average reading level of 8th grade for most U.S. adults, potentially hindering understanding and retention.
How can understanding and compliance with ICFs be improved?
Regular training sessions for research personnel on effective communication techniques can enhance understanding and compliance, fostering trust and contributing to more successful outcomes in clinical trials.
What is the importance of inclusivity in consent forms?
Streamlining consent forms to be inclusive and representative is not only a moral obligation but is also increasingly becoming a regulatory requirement in medical research.
Why is regulatory compliance crucial in clinical trials?
Maintaining regulatory compliance ensures that ethical standards are met, safeguarding participants and ensuring the integrity of the research data.
What services does bioaccess® provide to support regulatory compliance in clinical studies?
bioaccess® offers site feasibility, investigator selection, and strict regulatory adherence, including securing necessary approvals and managing import permits to comply with local regulations. Regular audits and training are also part of their comprehensive approach.