


This article provides essential insights into the regulatory requirements outlined in 21 CFR 807, which are paramount for clinical research directors in ensuring compliance and facilitating efficient market entry for medical devices. It highlights the necessity of:
to adeptly navigate the complexities of compliance. Such measures ultimately enhance operational efficiency and improve patient outcomes.
Navigating the intricate landscape of regulatory compliance is paramount for clinical research directors, particularly given the complexities outlined in 21 CFR 807. This regulation governs not only the establishment registration and device listing processes but also significantly impacts the timelines for introducing innovative medical products to the market. As the stakes rise, a comprehensive understanding of compliance strategies can unlock smoother approval pathways and enhance patient outcomes.
How can clinical research leaders effectively align their practices with these regulatory demands to ensure that both compliance and innovation flourish?
bioaccess® adeptly navigates the complexities of 21 CFR 807, empowering innovators in Medtech, Biopharma, and Radiopharma to secure regulatory approval efficiently. Leveraging its extensive regional expertise across Latin America, the Balkans, and Australia, bioaccess® aligns research initiatives with FDA regulations, significantly expediting the approval process and improving patient outcomes. This commitment to regulatory compliance is reinforced through comprehensive employee training on guidelines and regular audits to ensure adherence to the highest standards.
The impact of regulatory compliance on research timelines is significant; a well-structured compliance strategy streamlines processes and enhances the likelihood of successful market entry. bioaccess® provides a comprehensive suite of services, including:
All of which are crucial for accelerating research trials. Industry experts emphasize that presenting research data as a cohesive narrative is vital for improving approval prospects. Additionally, the recent extension of the Medical Device Regulation (MDR) transition period until 2027/2028 highlights the necessity of timely submissions, urging manufacturers to prepare their conformity assessment applications promptly to prevent market shortages.
In this dynamic environment, bioaccess® remains steadfast in guiding clients through the intricacies of 21 CFR 807, ensuring that their innovative products comply with 21 CFR 807 regulatory requirements while prioritizing positive patient outcomes. For clinical research directors, adopting a proactive stance by regularly assessing adherence strategies and incorporating real-world evidence into clinical evaluations is essential for enhancing the likelihood of successful market access.

Under the guidelines of 21 CFR 807, manufacturers are mandated to register their establishments with the FDA on an annual basis. This registration process, as outlined in 21 CFR 807, requires the submission of essential details, including the establishment's name, address, and the specific types of devices manufactured. Furthermore, any changes in ownership or location must be reported without delay to maintain compliance. Non-compliance can lead to significant penalties, including fines and potential delays in product approval, which can hinder market access and commercialization efforts.
To navigate these requirements effectively, clinical research directors should establish a robust tracking system that manages registration deadlines and updates. This proactive strategy ensures that all necessary information is submitted in a timely manner. It not only reduces risks linked to non-compliance but also facilitates smoother interactions with authorities and improves overall operational efficiency.
Have you considered how your current processes align with these regulatory demands?

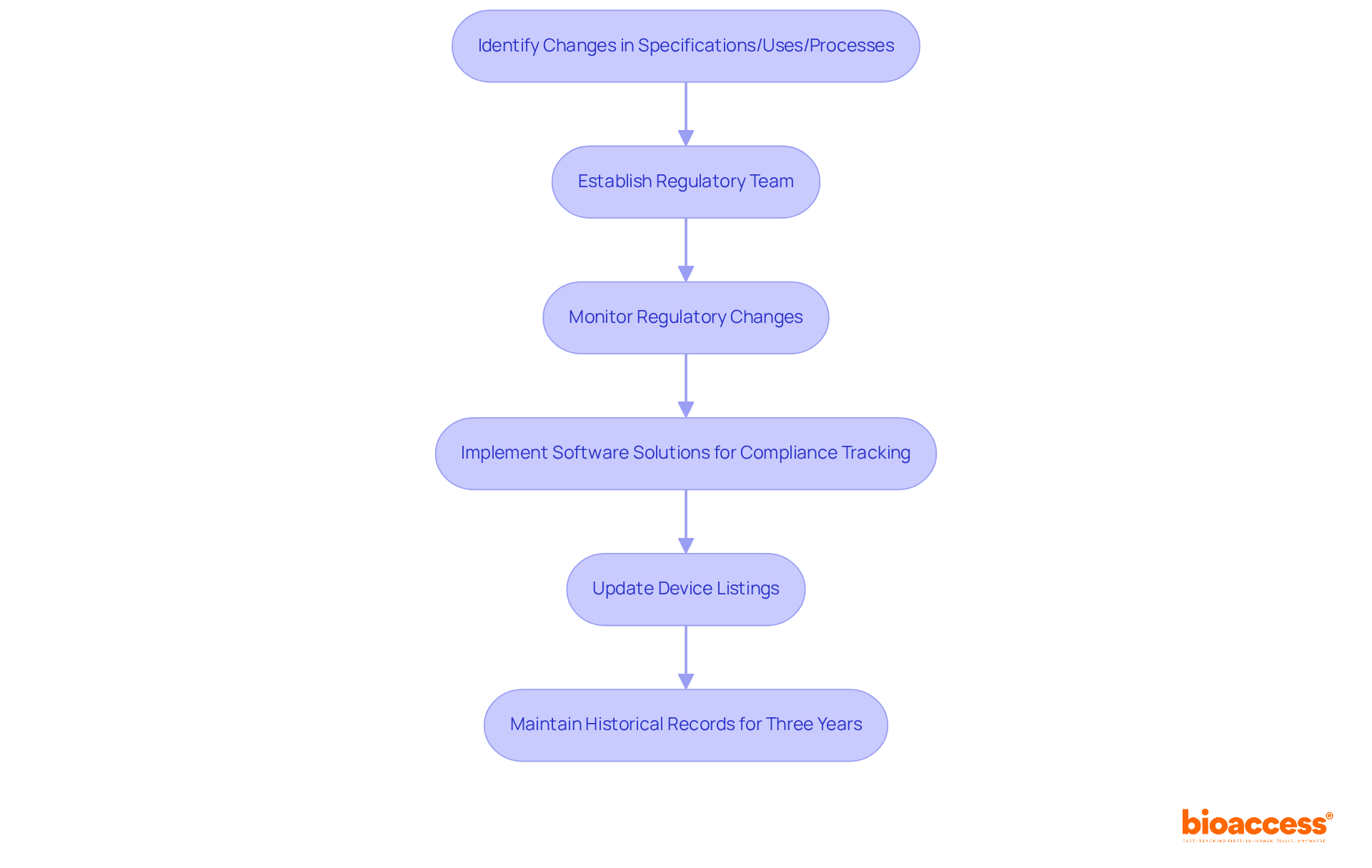
To ensure compliance with 21 CFR 807, manufacturers must promptly revise their product listings whenever there are changes in specifications, intended use, or manufacturing processes. Establishing a dedicated regulatory team is essential; this group should actively monitor regulatory changes and manage timely updates to product listings.
As Ana Criado, Director of Regulatory Affairs and a leading authority in the field, underscores, comprehending device classifications is vital for compliance, particularly since the FDA assigns a single listing number for all devices exempt from premarket notification requirements under a specific product code.
Implementing software solutions designed for FDA compliance tracking can greatly streamline this process, minimizing the risk of non-conformity. Viewing compliance as a strategic component, as noted by Criado, can convert challenges into opportunities for innovation and growth.
Timely updates not only facilitate smoother FDA approval processes—especially given the lengthy and intricate nature of pre-market approvals for higher-risk devices—but also enhance the overall integrity of product lifecycle management.
Furthermore, producers must maintain a historical record of labeling and advertisements for three years following the last shipment of a discontinued item, thereby ensuring compliance and promoting patient safety and product quality.
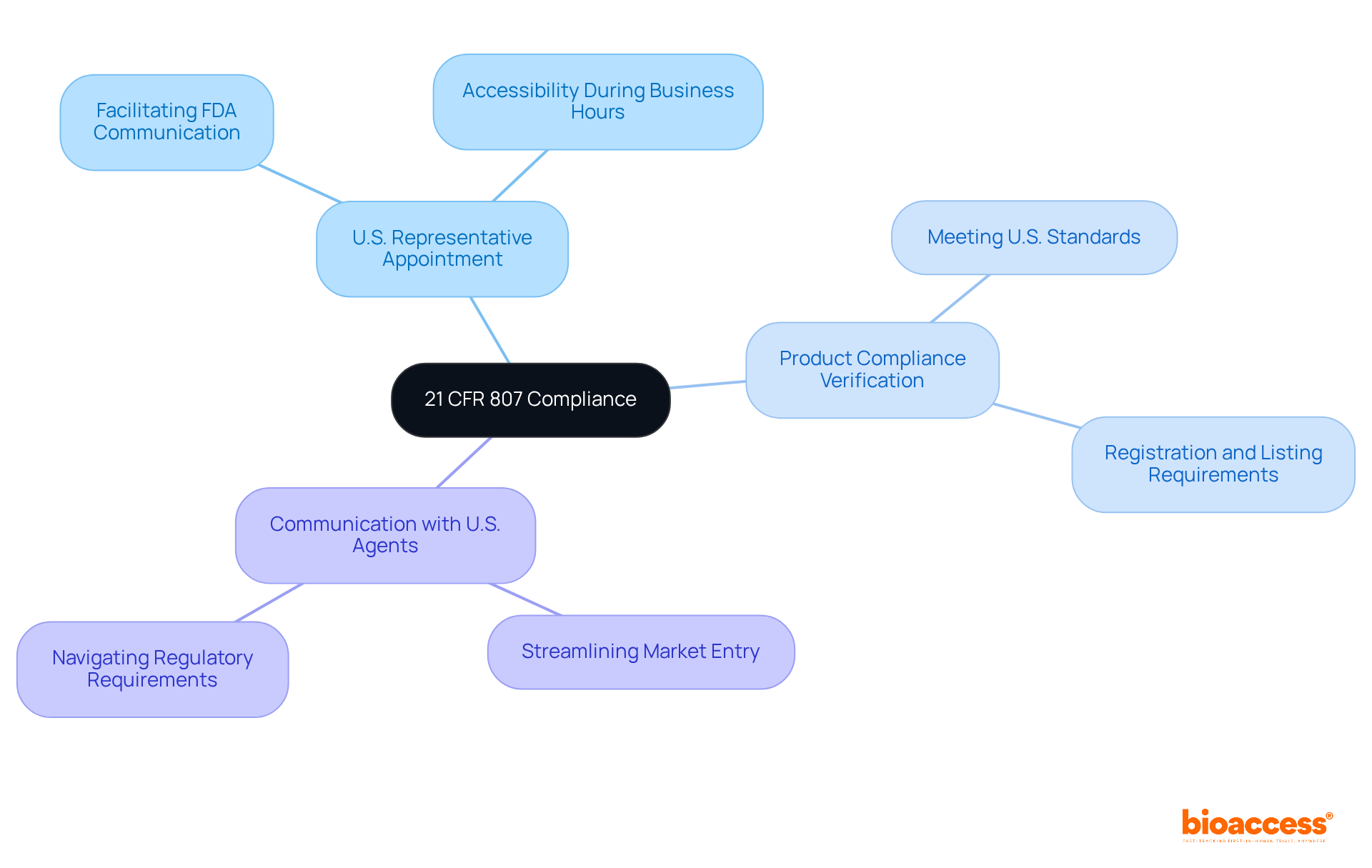
Foreign equipment establishments must comply with the regulations of 21 CFR 807 by appointing a U.S. representative to act on their behalf. This agent is pivotal in facilitating communication with the FDA and must be readily accessible during business hours to address any inquiries or compliance issues. Furthermore, it is essential for foreign companies to verify that their products meet U.S. compliance standards before market entry.
Clinical research directors should prioritize the establishment of robust communication channels with their U.S. agents, as this collaboration is vital for navigating regulatory requirements and ensuring prompt responses to any regulatory inquiries. By fostering this relationship, organizations can enhance their compliance initiatives and streamline the introduction of innovative medical products into the U.S. market.
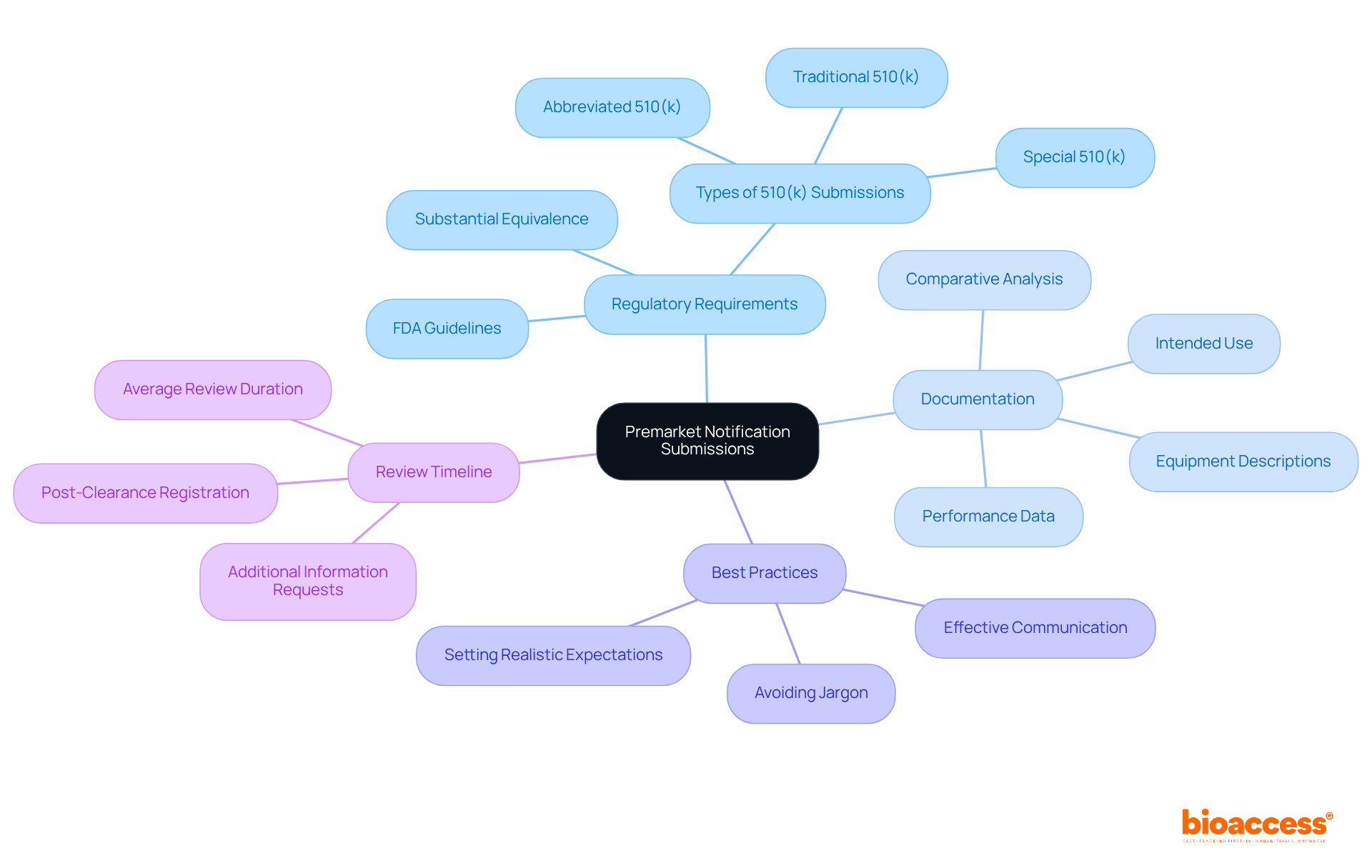
Premarket notifications, commonly referred to as 510(k) submissions, are mandatory for products that do not qualify for exemption from premarket review. Under the regulations of 21 CFR 807, manufacturers are required to demonstrate that their product is substantially equivalent to an existing legally marketed item. The average duration for a traditional 510(k) review is approximately 90 days; however, actual timelines may extend to six months or more due to requests for additional information.
To ensure a successful submission, it is critical to prepare comprehensive documentation that includes:
Regulatory experts emphasize the importance of a well-structured submission that articulates the product's intended use and technological features, as this significantly enhances the likelihood of a favorable review.
Examples of thorough documentation encompass:
Furthermore, insights from regulatory experts underscore that effective communication with the FDA, avoidance of jargon, and the establishment of realistic expectations for the review timeline are essential best practices. By prioritizing meticulous preparation and understanding the specific requirements outlined in 21 CFR 807, clinical research directors can effectively mitigate delays and streamline the approval process.
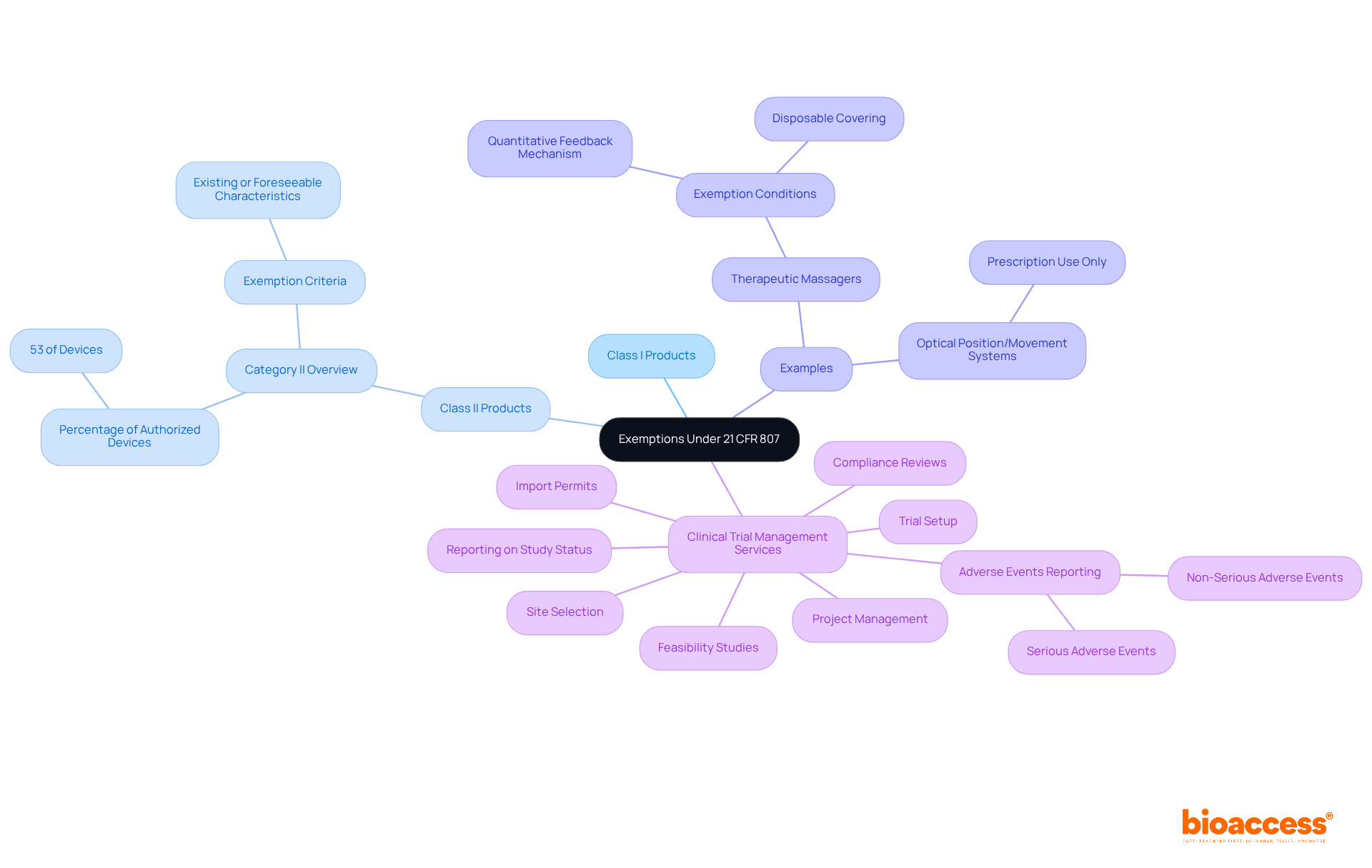
Under 21 CFR 807, certain items may qualify for exemption from the premarket notification requirements, particularly specific Class I and select Class II products. It is imperative for clinical research directors to understand the criteria for these exemptions, as they can substantially streamline the development process and lower submission costs. Approximately 53% of authorized devices fall under Category II, which encompasses many that may be exempt from premarket notifications.
For example:
Regularly reviewing the FDA's exemption list is essential for remaining informed about any changes, as the FDA is mandated to publish a notice at least once every five years. This proactive approach not only aids in compliance but also enhances the likelihood of successful market entry, as industry specialists emphasize the importance of aligning promotional strategies with compliance expectations.
At bioaccess, our comprehensive clinical trial management services include:
Under the guidance of Ana Criado, our Director of Compliance, who possesses extensive expertise in biomedical engineering and health economics, we ensure that our clients effectively navigate the complexities of compliance requirements.
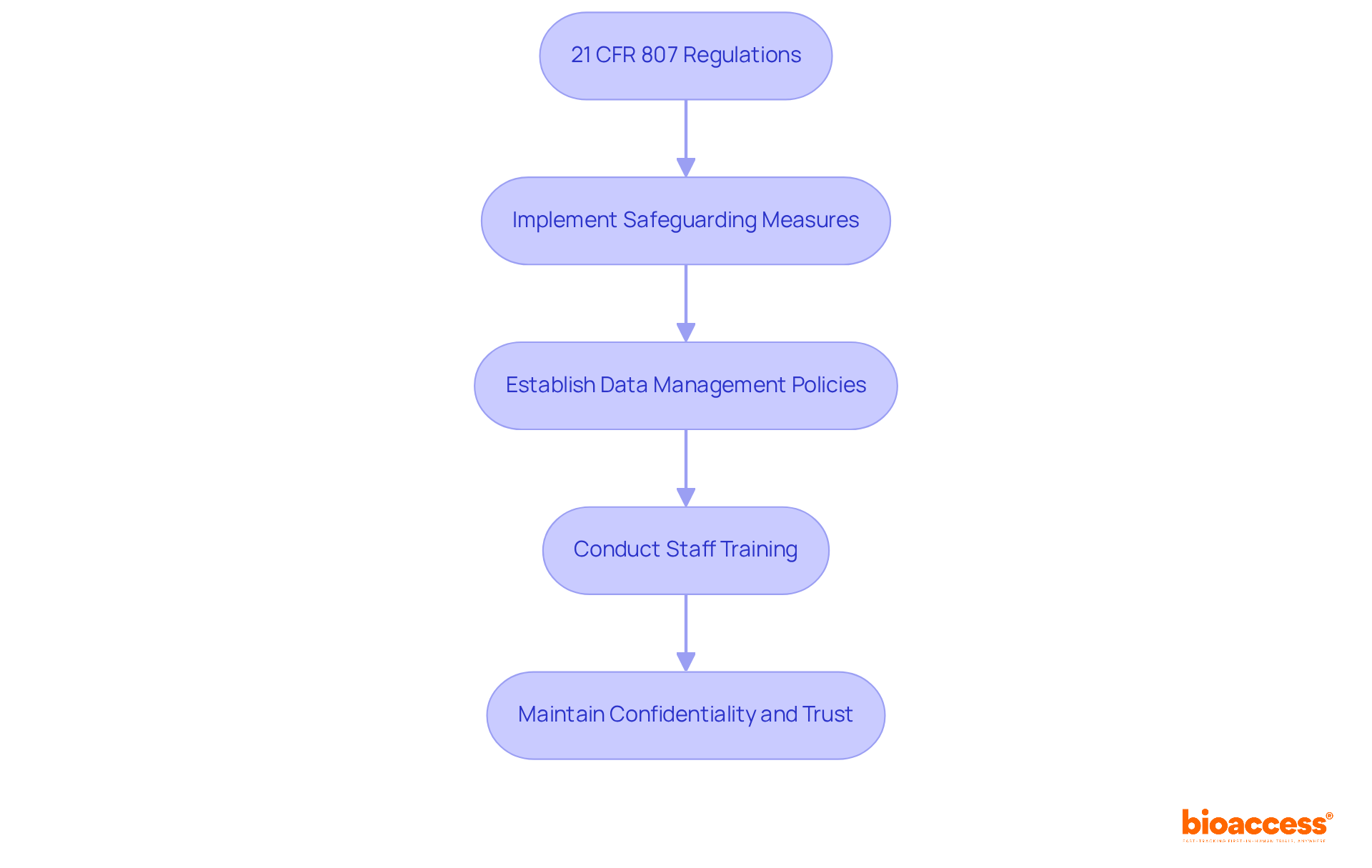
Under the regulations of 21 CFR 807, manufacturers are mandated to implement stringent measures to protect confidential information related to device registration and listings. Safeguarding proprietary data and ensuring that access to sensitive information is restricted to authorized personnel only is critical.
Clinical research directors must establish comprehensive data management policies that outline protocols for confidentiality and data protection. Regular training sessions for staff on these protocols are essential to mitigate the risks of data breaches, which can lead to significant financial and reputational harm.
As noted by industry experts, maintaining confidentiality is not merely a regulatory requirement; it is a cornerstone of trust in clinical research. By prioritizing robust data management practices, organizations can enhance their compliance and effectively protect sensitive information.
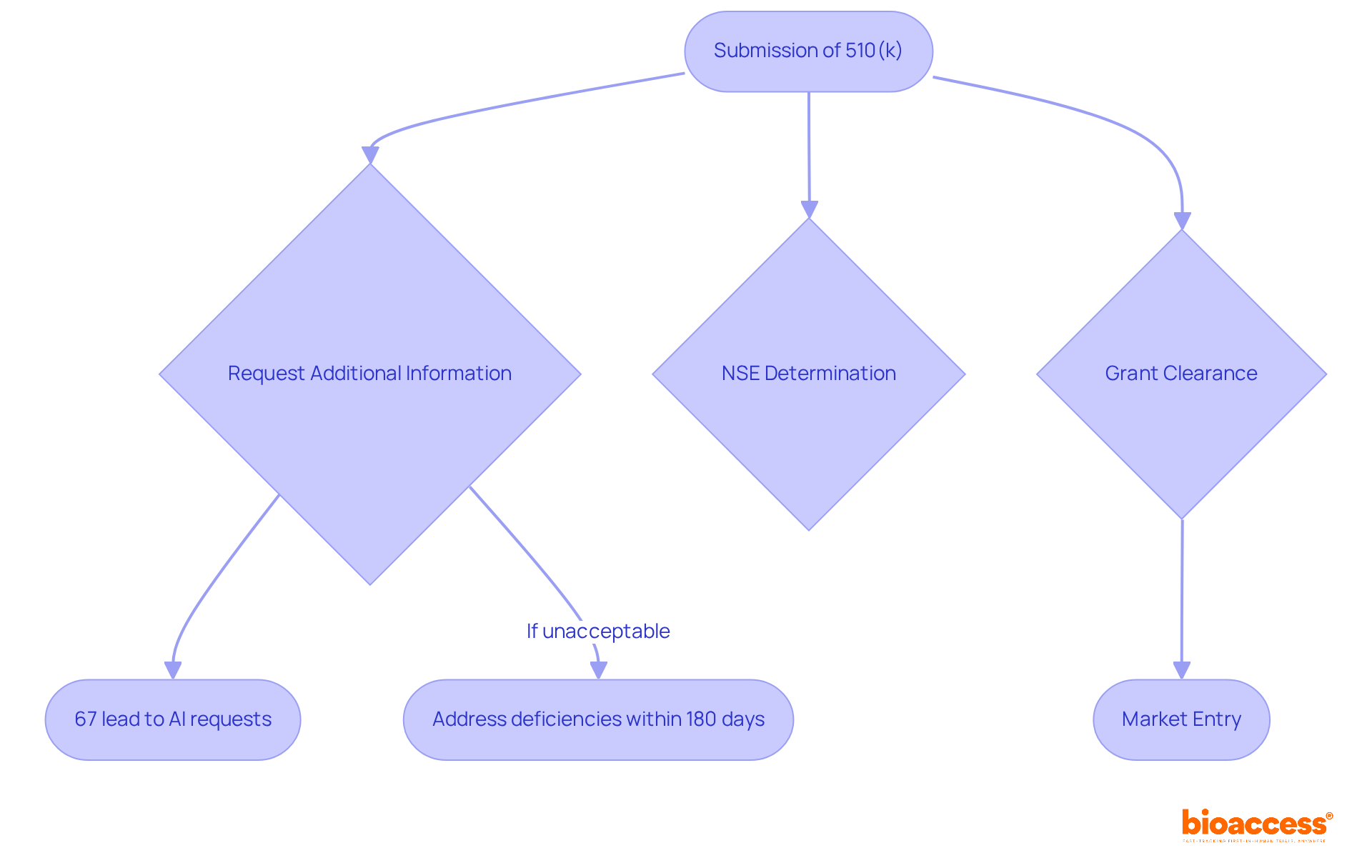
The FDA's actions regarding premarket notifications are pivotal in shaping the approval process for medical devices. Upon submission of a 510(k), the FDA may:
In 2021, the average time to receive a decision on 510(k) applications was approximately 147 days; however, this duration can extend to an average of five months due to requests for further information. Notably, 67% of submissions resulted in an Additional Information (AI) request, which can lead to further delays if not addressed promptly.
To effectively navigate these challenges, research directors must prioritize maintaining open lines of communication with the FDA. This involves preparing thorough submissions that meet all requirements, thereby minimizing the risk of NSE outcomes that can impede clinical research and market entry. NSE determinations may arise from significant variations in intended use or insufficient performance data compared to predicate products, making it essential to clearly demonstrate equivalence.
Effective communication strategies with the FDA often include proactive engagement, such as holding pre-submission meetings to clarify classification and expectations. Regulatory professionals assert that meticulous preparation can substantially reduce the likelihood of NSE outcomes, facilitating a more efficient approval process. By comprehending the implications of FDA actions and preparing accordingly, clinical research directors can pave smoother pathways to market for their innovative medical products.
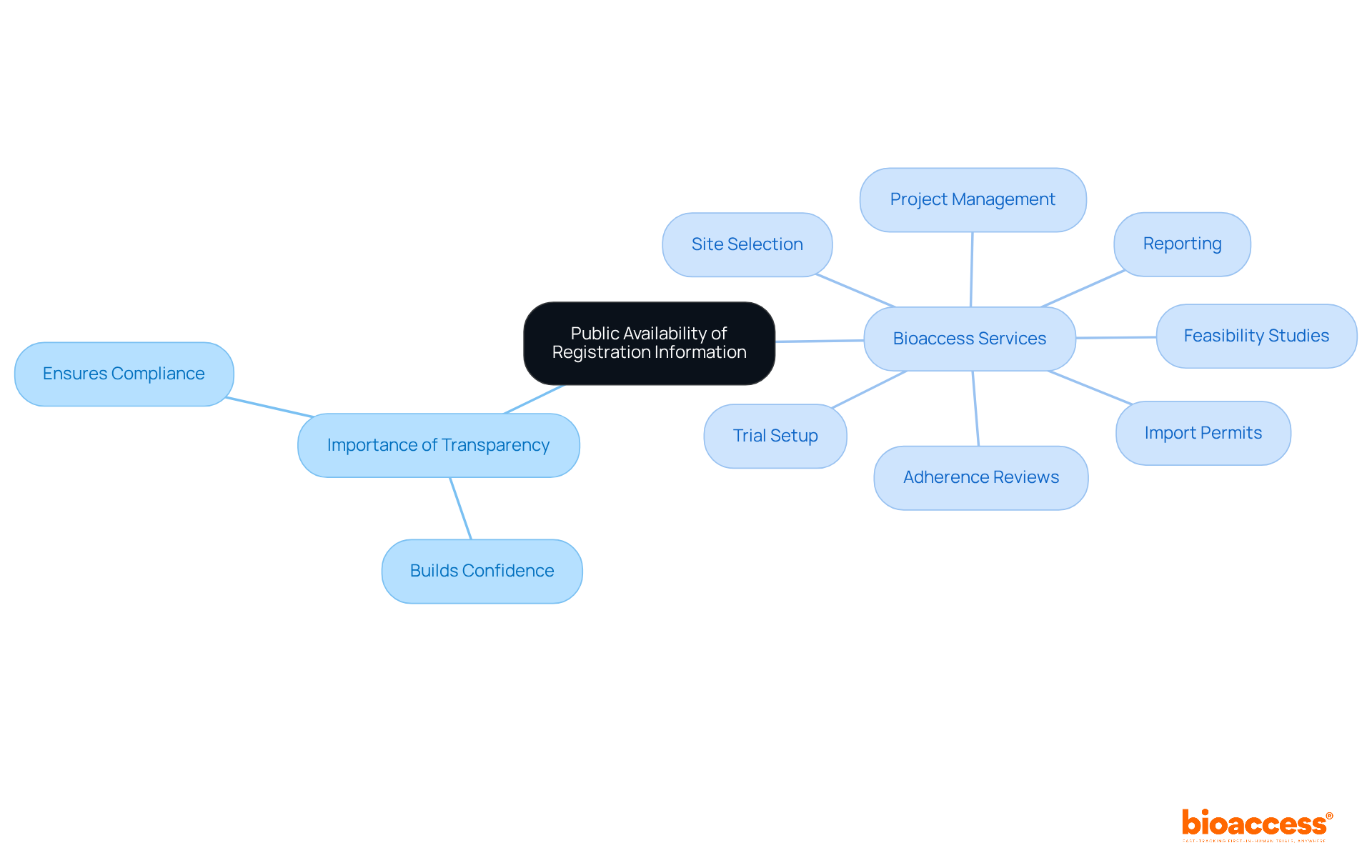
Under 21 CFR 807, it is required that establishment registration and device listing information be publicly available. This transparency fosters confidence in the oversight process and empowers stakeholders to verify compliance. Clinical research leaders must ensure their registration details are accurate and up-to-date; discrepancies can lead to reputational damage and regulatory scrutiny.
Bioaccess offers comprehensive trial management services, including:
Regular assessments of public information can help uphold standards, and leveraging Bioaccess's expertise can mitigate the challenges faced by medical device startups in navigating legal complexities.
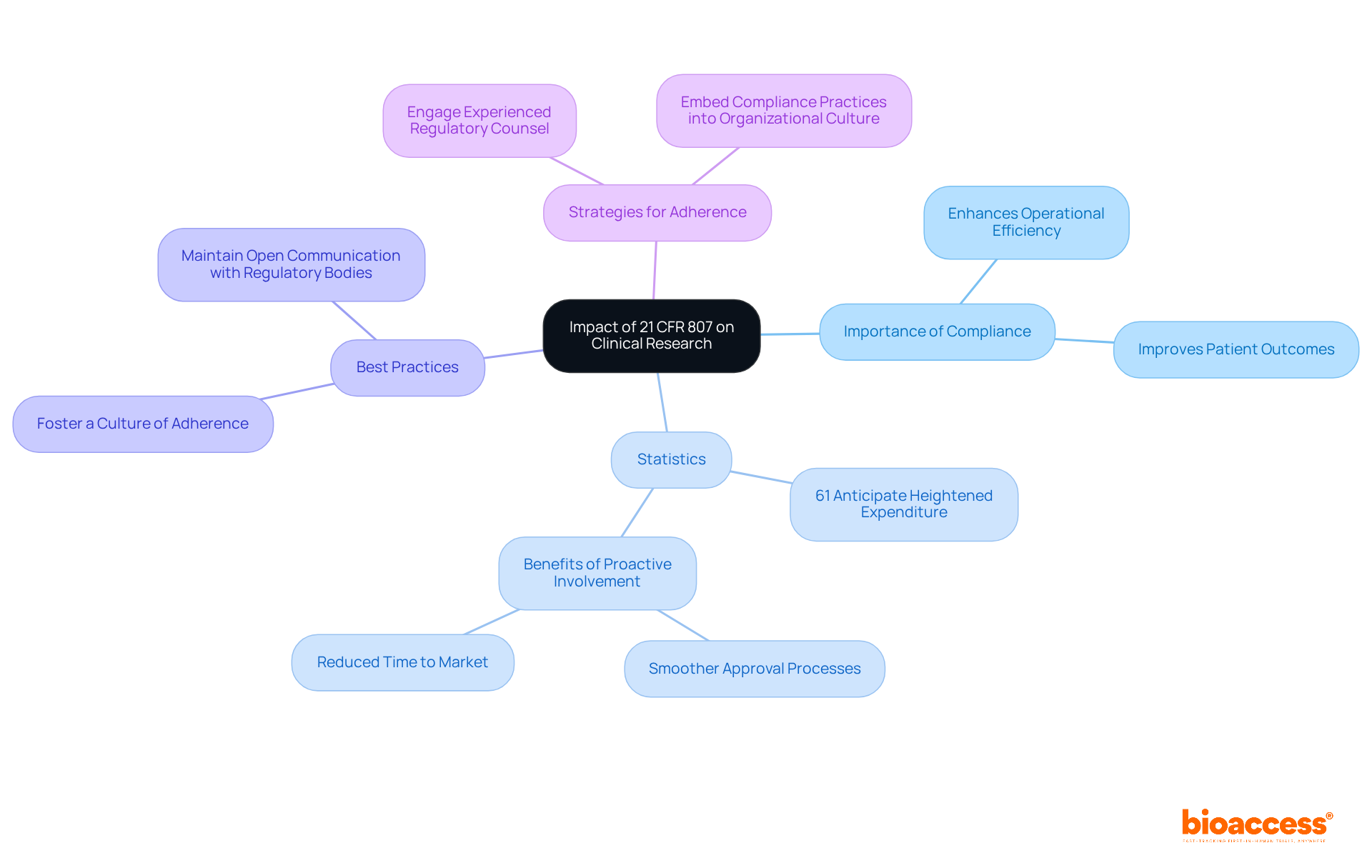
The regulations in 21 CFR 807 are pivotal in shaping clinical research practices, encompassing everything from establishment registration to premarket notifications. Adhering to these regulations is crucial for successful product development and market entry.
Clinical research directors must remain vigilant regarding regulatory changes and adopt best practices to effectively manage the intricacies of adherence. Organizations that prioritize a culture of adherence often report enhanced operational efficiency and improved patient outcomes.
Statistics reveal that:
Industry leaders emphasize that maintaining open communication with regulatory bodies and fostering a robust adherence framework are essential strategies for overcoming regulatory challenges. By embedding these practices into their organizational culture, clinical research directors can significantly enhance their teams' ability to navigate the complexities of compliance with 21 CFR 807.
Navigating the complexities of 21 CFR 807 is essential for clinical research directors seeking to ensure compliance and streamline the approval process for medical devices. This regulation mandates rigorous adherence to registration and listing requirements, emphasizing the importance of a proactive approach to regulatory changes and communication with the FDA. By understanding and implementing these guidelines, organizations can significantly enhance their operational efficiency and improve patient outcomes.
Key insights from the article highlight critical components of 21 CFR 807, including the necessity of:
The significance of maintaining confidentiality and ensuring public availability of registration information further underscores the responsibilities of clinical research directors. By adopting best practices and leveraging resources such as bioaccess®, organizations can effectively navigate these regulatory landscapes and mitigate potential risks.
Ultimately, adherence to 21 CFR 807 is not merely a compliance obligation; it is a strategic advantage that can lead to successful market entry and innovation in medical technology. Clinical research directors are encouraged to continuously assess their compliance strategies, foster open communication with regulatory bodies, and stay informed about evolving regulations. This proactive stance not only safeguards their organizations but also contributes to the overarching goal of delivering safe and effective medical products to patients in need.
What is bioaccess® and how does it assist with 21 CFR 807 compliance?
bioaccess® helps innovators in Medtech, Biopharma, and Radiopharma navigate the complexities of 21 CFR 807, enabling them to secure regulatory approval efficiently. It leverages regional expertise and aligns research initiatives with FDA regulations to expedite the approval process and improve patient outcomes.
What services does bioaccess® provide to accelerate research trials?
bioaccess® offers a comprehensive suite of services, including feasibility studies, investigator selection, trial management, import permits, nationalization of investigational devices, and reporting on study status and adverse events.
Why is regulatory compliance important for research timelines?
A well-structured compliance strategy streamlines processes and enhances the likelihood of successful market entry, thereby significantly impacting research timelines.
What are the key requirements for device establishment registration under 21 CFR 807?
Manufacturers must register their establishments with the FDA annually, submitting essential details like the establishment's name, address, and types of devices manufactured. Changes in ownership or location must also be reported promptly to maintain compliance.
What are the consequences of non-compliance with 21 CFR 807?
Non-compliance can lead to significant penalties, including fines and potential delays in product approval, which can hinder market access and commercialization efforts.
How can clinical research directors effectively manage registration deadlines and updates?
Establishing a robust tracking system to manage registration deadlines and updates ensures timely submission of necessary information, reduces risks linked to non-compliance, and facilitates smoother interactions with authorities.
What best practices should manufacturers follow for updating device listings under 21 CFR 807?
Manufacturers must promptly revise their product listings whenever there are changes in specifications, intended use, or manufacturing processes. Establishing a dedicated regulatory team to monitor changes and manage updates is essential.
How can software solutions aid in compliance with FDA regulations?
Implementing software solutions designed for FDA compliance tracking can streamline the process of managing updates and minimize the risk of non-conformity.
What historical records must producers maintain for compliance?
Producers must maintain a historical record of labeling and advertisements for three years following the last shipment of a discontinued item to ensure compliance and promote patient safety and product quality.