


This article examines the costs associated with medical device registration services in Mexico, underscoring that these costs fluctuate based on the classification of the device and the complexity of the application process. Registration fees typically fall within the range of $500 to $1,250 USD, contingent upon the device class. It is crucial to grasp the additional expenses tied to documentation and compliance, as this understanding is vital for effectively budgeting for market entry.
The medical device industry is witnessing remarkable growth, particularly in emerging markets such as Mexico, where the healthcare equipment sector is anticipated to reach a staggering $6.25 billion. However, the complexities surrounding medical device registration can pose significant challenges for manufacturers, especially when factoring in the costs related to compliance and approval processes.
This article explores the intricacies of medical device registration services in Mexico, offering essential insights into:
What strategies can manufacturers implement to streamline their registration processes and enhance their chances of success in this lucrative market?
bioaccess® leverages its extensive regional expertise to expedite the medical device registration services Mexico cost, allowing for health product approvals in as little as 4-6 weeks. This rapid turnaround starkly contrasts with conventional trading environments, where timelines can extend considerably. Such agility is critical for Medtech innovators aiming to swiftly introduce their products while ensuring compliance with COFEPRIS regulations, which includes understanding the medical device registration services Mexico cost and adherence to NOM-241-SSA1-2021, governing healthcare instruments in Mexico.
By harnessing local expertise and regulatory insights, bioaccess® not only minimizes delays but also enhances the likelihood of successful market entry. Our comprehensive clinical trial management services encompass:
This ensures a streamlined process. As the second-largest healthcare equipment market in Latin America, valued at approximately $6.25 billion in 2022, Mexico presents a lucrative opportunity. Companies that prioritize regulatory efficiency and comply with essential requirements, such as obtaining a COFEPRIS sanitation license and monitoring adverse events, are strategically positioned to capitalize on the potential offered by medical device registration services Mexico cost.
Engaging with local regulatory experts can provide Medtech companies with invaluable guidance on COFEPRIS regulations, streamlining the registration process and minimizing errors. With bioaccess®, you can accelerate your clinical trials and navigate the complexities of regulatory approvals with confidence.
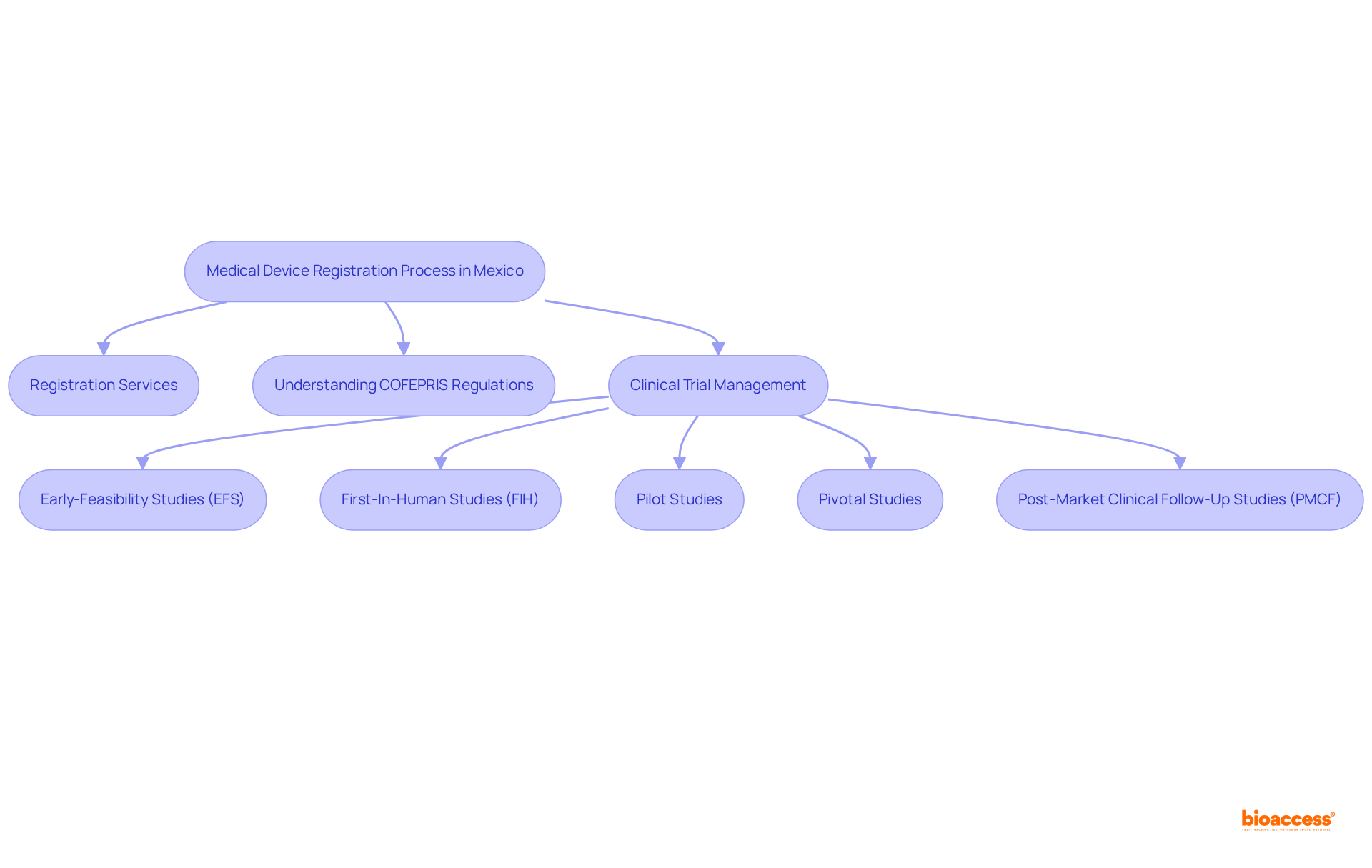
The Federal Commission for Protection against Sanitary Risks (COFEPRIS) serves as the cornerstone of healthcare equipment regulation in Mexico, operating under the Ministry of Health. This agency is tasked with ensuring that all healthcare instruments meet stringent safety, effectiveness, and quality criteria before they enter the marketplace. Each year, COFEPRIS processes thousands of healthcare equipment applications, underscoring its vital role in the health sector.
Regulatory specialists emphasize that compliance with COFEPRIS standards is essential for producers aiming to navigate the complexities of the Mexican business landscape effectively, especially regarding medical device registration services Mexico cost. Through comprehensive evaluations, inspections, and the enforcement of health regulations, COFEPRIS not only safeguards public health but also fosters a reliable environment for healthcare innovation. Its commitment to maintaining high standards is crucial for ensuring the safety of healthcare products, thereby enhancing consumer confidence and facilitating market entry for producers.
In this context, collaborating with bioaccess® can provide invaluable support through extensive clinical trial management services and medical device registration services Mexico cost, including:
All designed to ensure that your products efficiently meet COFEPRIS standards. Katherine Ruiz, a specialist in regulatory matters for healthcare products and in vitro diagnostics in Colombia, further highlights the importance of having informed support in navigating these regulatory landscapes. To optimize your chances of successful market entry, consider engaging with bioaccess® for customized assistance in your clinical trial requirements.
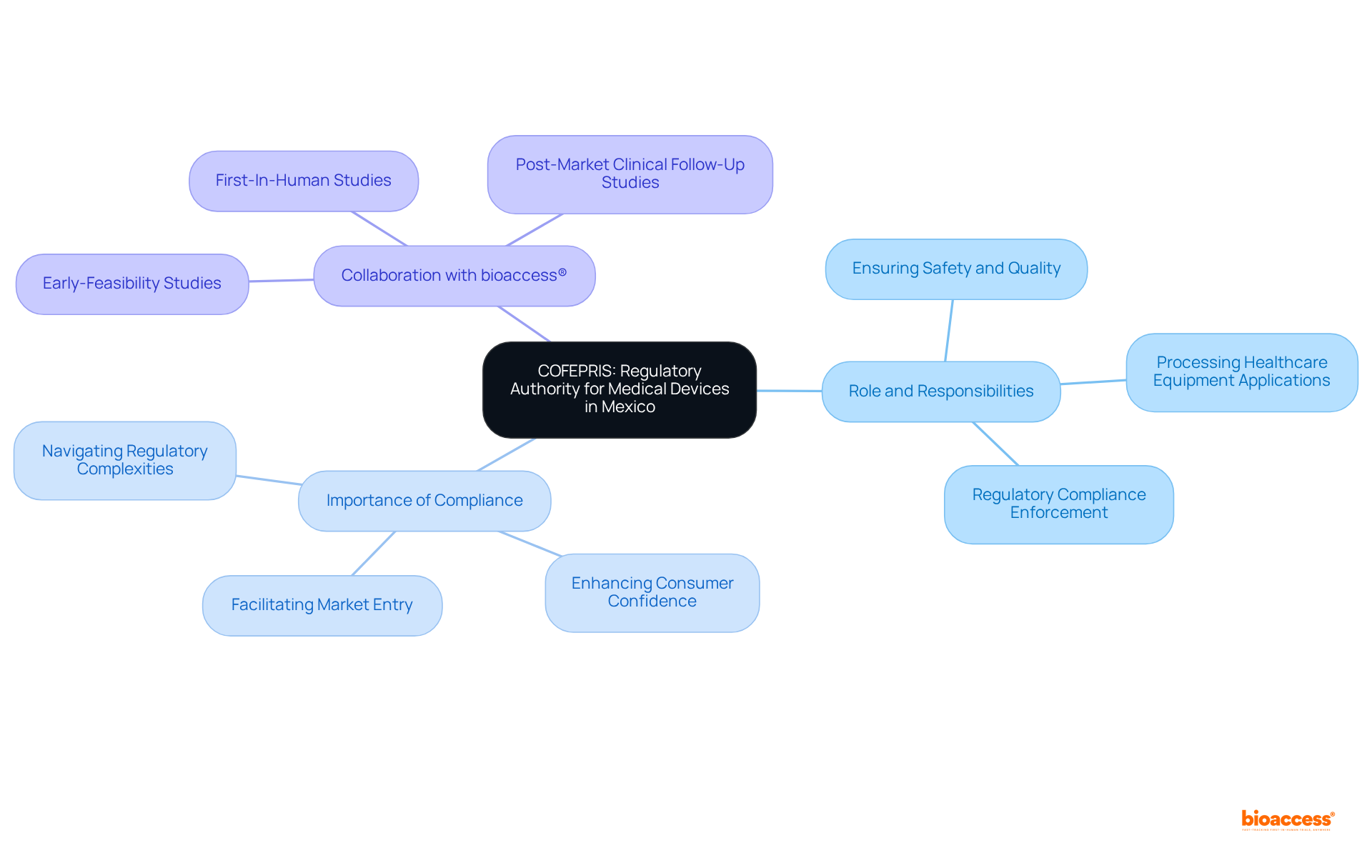
The registration process for medical devices in Mexico is a structured procedure that involves several critical steps:
Classification: The first step is to determine the classification of the equipment based on its risk level, which can be categorized as Class I, II, or III. This classification is essential as it dictates the regulatory requirements that follow.
Documentation: Next, manufacturers must prepare comprehensive documentation. This encompasses technical documents, quality management system certifications, and pertinent clinical information that illustrate the safety and effectiveness of the product. Significantly, producers must provide evidence of an examined quality system as part of their application process, except for specific low-risk items.
Submission: Once the documentation is complete, the application for enrollment is submitted to COFEPRIS, the regulatory authority in Mexico, along with the required application fees.
Review: COFEPRIS conducts a thorough review of the application. During this phase, additional information may be requested to ensure compliance with regulatory standards. As highlighted by industry leaders, understanding the nuances of local regulations is crucial, as each country has its own specific requirements that can impact the approval process.
Approval: Upon successful review, COFEPRIS issues a registration certificate, which allows the product to be marketed in Mexico. This procedure usually requires 4 to 6 weeks, considerably quicker than numerous conventional sectors, facilitating faster entry to the expanding healthcare equipment sector in Mexico, which is anticipated to achieve a revenue of US$3,226.2 million by 2030.
Understanding these steps is crucial for manufacturers aiming to navigate the regulatory landscape of medical device registration services Mexico cost effectively. Successful instances of healthcare apparatus classification and documentation have shown that comprehensive preparation can result in accelerated approvals, enabling prompt entry into the commercial sector. As industry leaders emphasize, "Preparation is key to navigating the complexities of the COFEPRIS application process effectively." Additionally, leveraging local expertise and adapting strategies to meet specific market needs can enhance the chances of successful market access in Mexico.
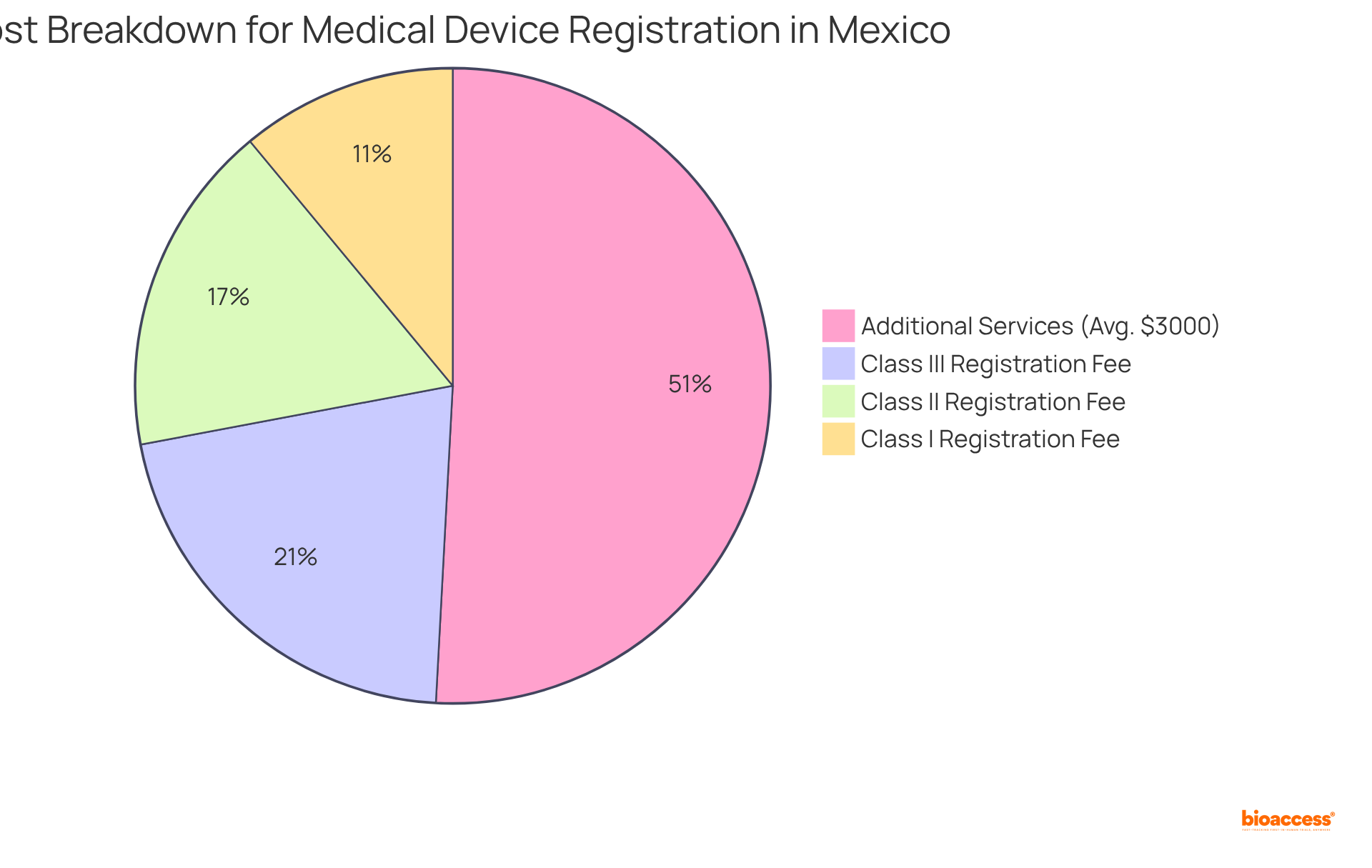
The medical device registration services Mexico cost is significantly influenced by the classification of the equipment and the complexity of the application process. The medical device registration services Mexico cost typically ranges from $500 to $1,100 USD, depending on the equipment's classification. For instance, the medical device registration services Mexico cost for Class I items is approximately $650 USD, while Class II and Class III items incur charges of $1,000 USD and $1,250 USD, respectively. Additionally, manufacturers may need to hire third-party reviewers, and the medical device registration services in Mexico cost can vary from $2,000 to $4,000 USD, depending on the reviewer and device class.
Beyond these enrollment fees, it is essential to account for additional expenses like medical device registration services Mexico cost that can significantly impact the overall budget for market entry. These may include costs related to:
By utilizing bioaccess's clinical trial management services, manufacturers can streamline the process through:
As the regulatory landscape evolves, staying informed about current trends in medical device registration services Mexico cost is crucial for effective financial planning. For example, the 2024 fees reflect an increase, with Class I enrollment now set at MXN 15,206, Class II at MXN 22,302, and Class III at MXN 28,384.
It is vital to recognize that the medical device registration services in Mexico cost includes approval that is valid for five years, with the option for renewal for an additional five years—an important consideration for manufacturers in their long-term budgeting. Furthermore, adherence to COFEPRIS’s Good Manufacturing Practices (GMP) is critical, as inspections may be conducted to verify compliance with these standards.
Grasping these financial obligations is essential for manufacturers aiming to adeptly navigate the complexities of the Mexican market, particularly regarding medical device registration services Mexico cost. Furthermore, manufacturers should be aware that unused payments can be refunded by submitting proof to COFEPRIS through SAT. Expert guidance on budgeting for COFEPRIS fees and related expenses, particularly through the lens of bioaccess's clinical trial management services, can facilitate the application process and mitigate potential financial risks. To effectively budget for these fees, manufacturers should consider consulting with regulatory experts who can offer tailored advice based on their unique circumstances.
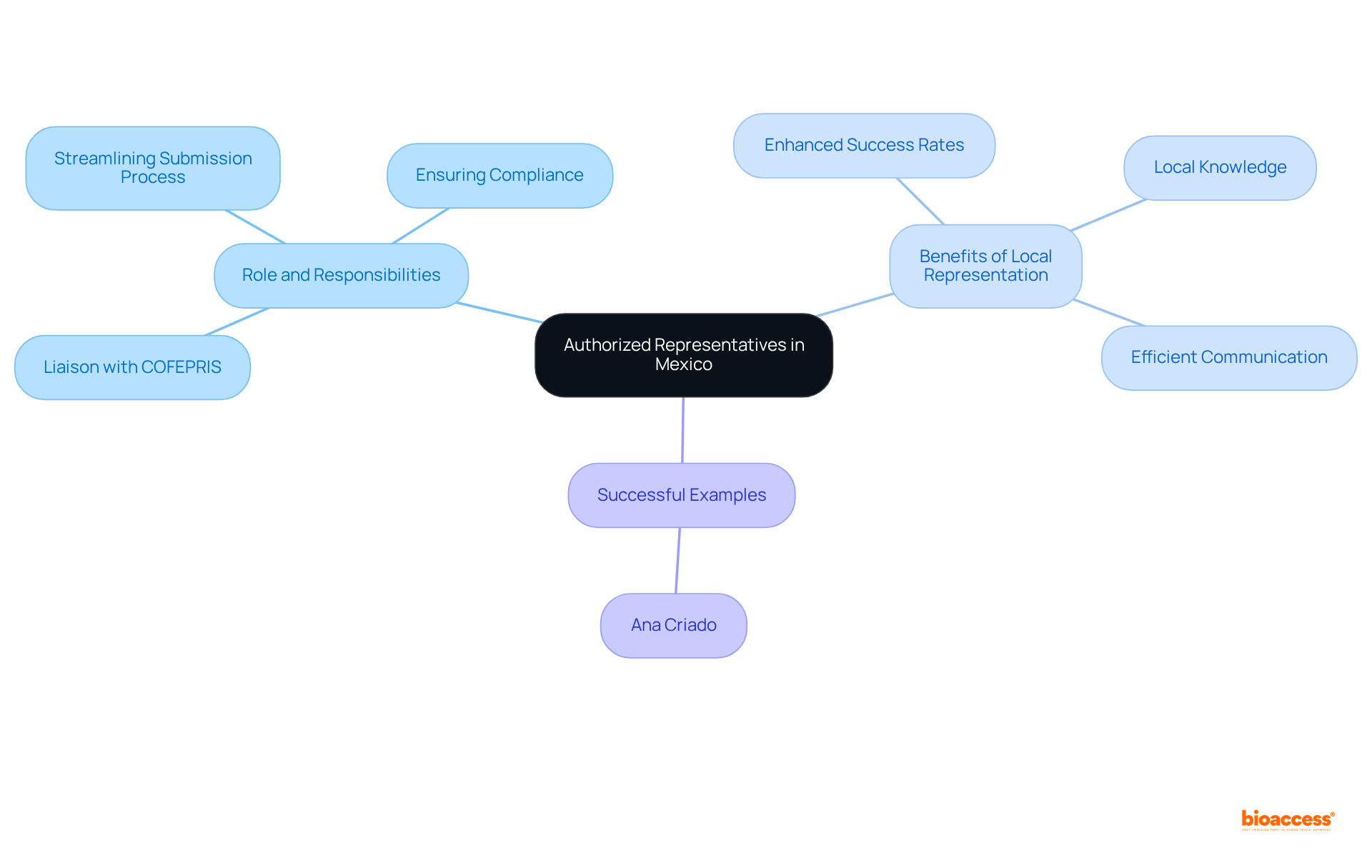
To successfully navigate the medical device certification process in Mexico, foreign manufacturers must appoint a local Authorized Representative, known as a Mexico Registration Holder (MRH). This MRH serves as the primary liaison between the manufacturer and COFEPRIS, the regulatory authority, ensuring efficient communication and compliance with local health regulations. Such a local presence is crucial for addressing the complexities of the enrollment process, including regulatory inquiries and adherence to Mexican health laws.
The importance of local representation cannot be overstated. Regulatory consultants emphasize that having an MRH significantly enhances the likelihood of successful registration. These representatives provide invaluable insights into the nuances of the regulatory landscape, often challenging for foreign manufacturers unfamiliar with local requirements. For instance, an experienced MRH can streamline the submission process, ensuring that all necessary documentation is accurately prepared and submitted promptly.
Numerous successful examples illustrate how effective local representation has led to expedited approvals for medical devices. Firms that have engaged knowledgeable MRHs report greater success rates in navigating the regulatory maze, ultimately reducing time to market. Furthermore, local representatives play a vital role in ensuring compliance after registration, which is essential for maintaining access in Mexico.
In this context, professionals like Ana Criado, with her extensive background in regulatory affairs and experience with global companies, exemplify the type of expertise that can significantly benefit foreign manufacturers. Her qualifications in health economics and regulatory practices provide critical insights into the Mexican industry, enhancing the effectiveness of the MRH.
In summary, appointing a local MRH is not merely a regulatory requirement; it is a strategic action that greatly influences the success of health product approval in Mexico. By leveraging local knowledge, such as that provided by experts like Ana Criado, manufacturers can improve their prospects for a seamless and effective enrollment process, paving the way for successful market entry.
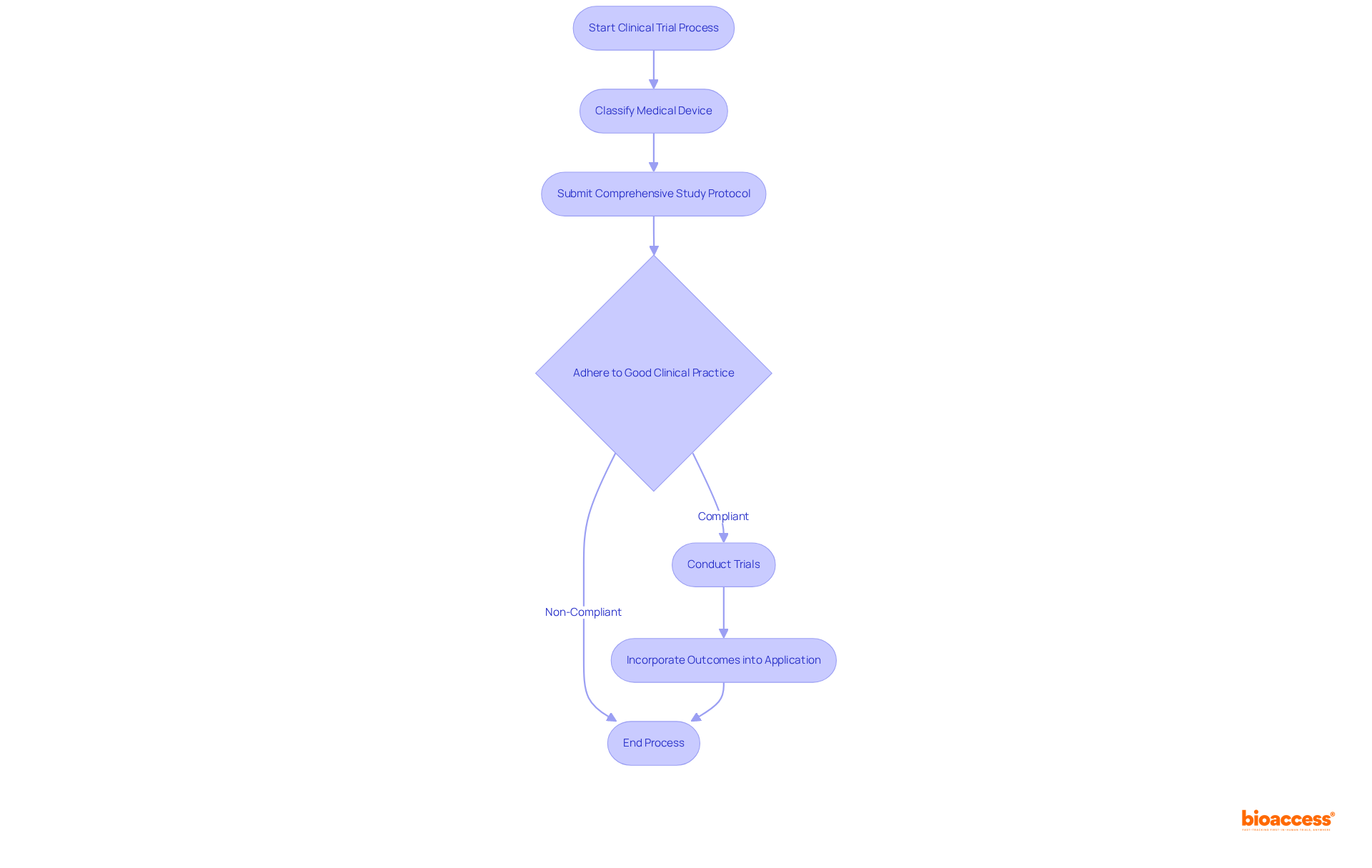
For high-risk healthcare instruments classified as Class III, conducting clinical trials is a prerequisite to demonstrate safety and efficacy prior to approval. Notably, approximately 10% of medical equipment submissions in Mexico fall under this category, necessitating rigorous testing protocols.
Manufacturers must submit a comprehensive study protocol to COFEPRIS, detailing the trial design, objectives, methodology, and participant recruitment strategies. Adherence to Good Clinical Practice (GCP) guidelines is crucial, as these standards ensure the ethical and scientific quality of trials. The outcomes from these trials must be incorporated into the application to substantiate the device's safety profile.
Recent regulatory updates have streamlined the approval process, allowing for more efficient navigation of clinical trial protocols, which is essential for timely market entry. Collaborating with knowledgeable partners, such as bioaccess®, can enhance compliance and expedite the approval process. Bioaccess® connects innovative Medtech, Biopharma, and Radiopharma startups with top-ranked clinical research sites, ensuring trials can commence 40% faster.
To maximize your chances of successful commercialization, consider leveraging bioaccess®'s expertise in navigating the clinical trial landscape.
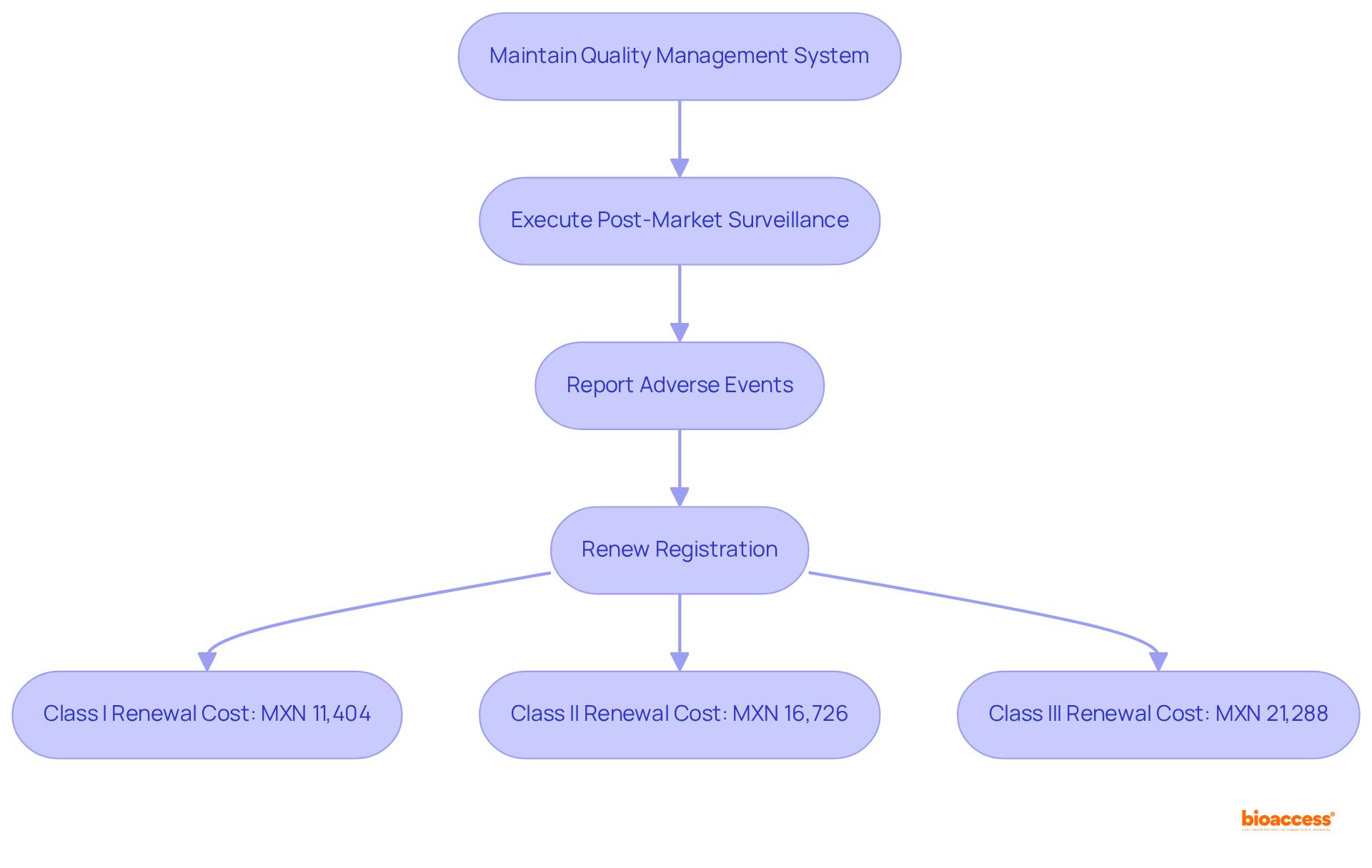
Once a medical instrument secures registration from COFEPRIS, manufacturers face several critical post-registration responsibilities. These responsibilities encompass:
In 2024, the medical device registration services Mexico cost for renewing Class I, II, and III healthcare instruments are set at:
Adhering to these responsibilities is vital for ensuring the ongoing safety and effectiveness of health products. Regulatory specialists emphasize the importance of post-market monitoring in maintaining public confidence and ensuring continued product reliability. Engaging with local compliance experts, such as those from bioaccess®, can significantly streamline this process, offering invaluable insights into COFEPRIS's evolving requirements and enhancing manufacturers' capabilities to navigate the regulatory landscape effectively. Furthermore, maintaining clinical records is essential for compliance, guaranteeing that all necessary documentation is readily available for review and audit purposes.
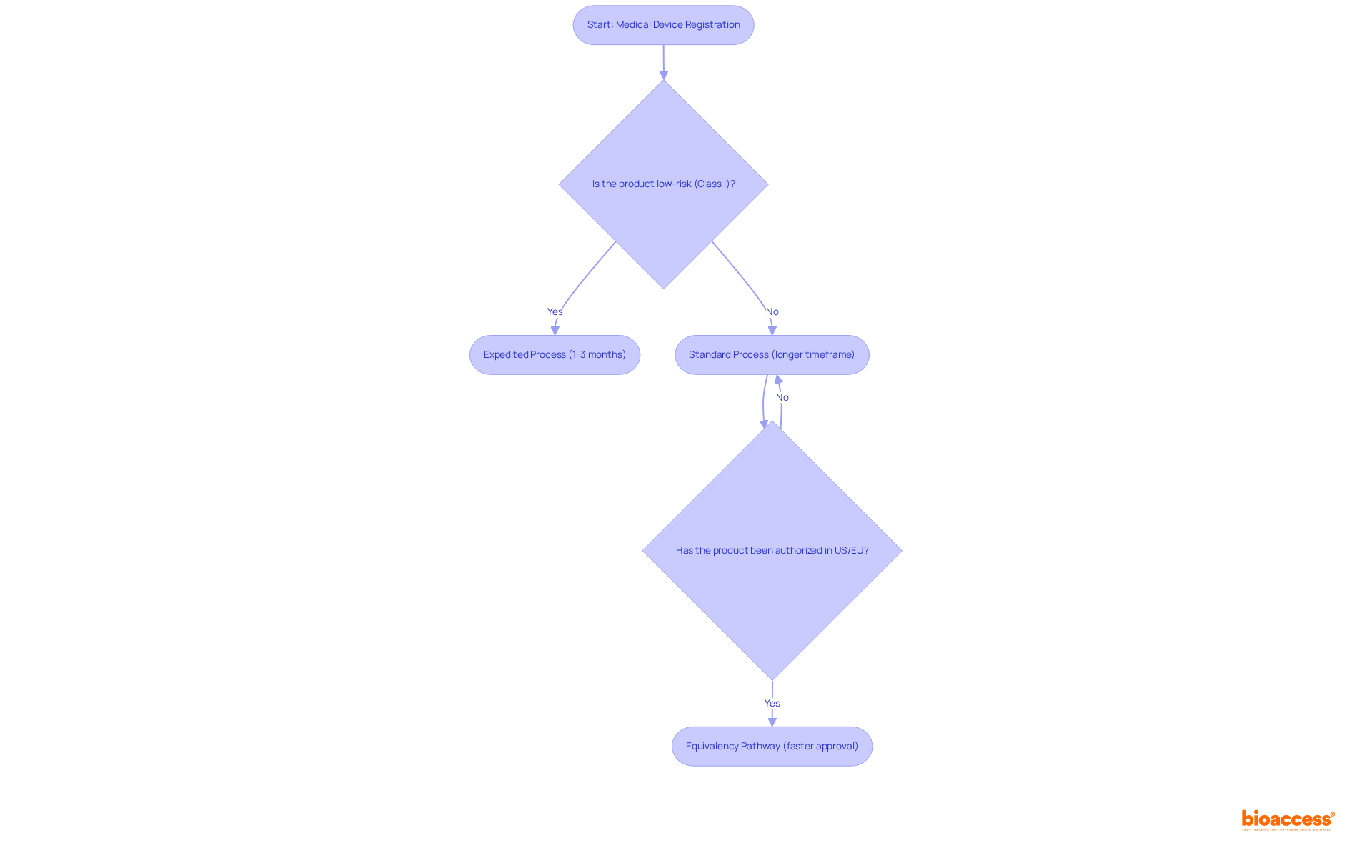
COFEPRIS has established exemptions and accelerated procedures specifically for low-risk medical products (Class I), which often do not necessitate complete documentation. This streamlined process for medical device registration services in Mexico allows for the efficient registration of these devices, significantly reducing both time and expenses. For instance, low-risk products typically receive approval in just 1 to 3 months, a stark contrast to the protracted timelines associated with higher-risk categories.
Furthermore, devices that have previously been authorized in countries with stringent regulatory standards, such as the US or EU, may qualify for the equivalency pathway. This pathway enables even swifter approval in Mexico by leveraging the established trust in these international endorsements. Producers are strongly encouraged to collaborate closely with their authorized representatives to explore these expedited options and confirm their eligibility, thereby ensuring a smoother entry into the Mexican market.
In Mexico, the registration of software and digital health applications is governed by specific technical standards established by COFEPRIS. These standards require that products demonstrate reliability, security, and compliance with data protection regulations. Manufacturers must submit comprehensive documentation detailing the software's intended use, functionality, and clinical evidence supporting its efficacy.
As of 2025, the number of registered digital health applications in Mexico is anticipated to rise significantly, reflecting the sector's rapid expansion. Adhering to COFEPRIS standards is essential for developers aiming to penetrate the Mexican market, particularly in light of the increasing demand for digital health solutions.
Experts in the field emphasize that compliance with these standards not only facilitates market entry but also bolsters consumer trust in digital health products. Staying informed about regulatory changes is crucial for success in this evolving landscape.
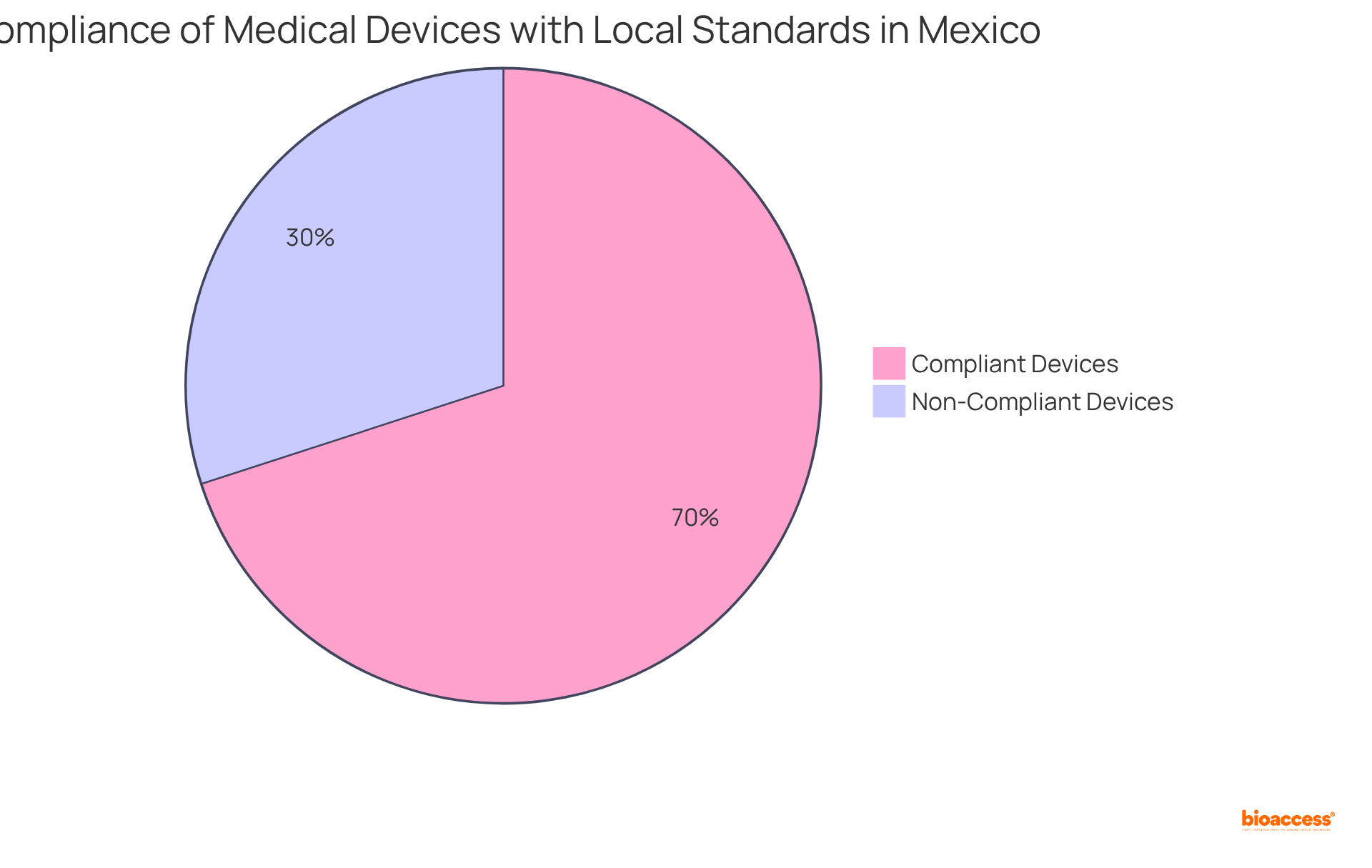
In Mexico, adherence to local standards is essential for healthcare products, as they must follow specific testing criteria beyond COFEPRIS regulations. Notably, a considerable portion of equipment—approximately 30%—does not meet local standards testing, underscoring the necessity of comprehensive preparation.
Manufacturers are mandated to conduct electrical safety testing in accordance with IEC standards while also meeting performance criteria established by NOMs (Official Mexican Standards). Recent updates to NOMs have introduced stricter guidelines, necessitating that manufacturers collaborate closely with their authorized representatives to navigate these requirements effectively.
This proactive approach is vital for successful medical device registration services Mexico cost and entry into Mexico's rapidly growing medical device sector.
Understanding the intricacies of medical device registration services in Mexico is essential for manufacturers aiming to penetrate this lucrative market. This article highlights the critical role that local expertise, particularly from organizations like bioaccess®, plays in expediting the registration process while ensuring compliance with COFEPRIS regulations. By leveraging regional knowledge and streamlined clinical trial management, companies can significantly reduce their time to market and enhance their chances of successful product approval.
Key insights discussed include:
Engaging with authorized representatives not only simplifies communication with regulatory authorities but also boosts the likelihood of navigating the complex landscape effectively. Furthermore, understanding the specific requirements for low-risk products and the implications of local standards is crucial for manufacturers aiming to achieve compliance and market entry.
In conclusion, the pathway to successful medical device registration in Mexico is paved with strategic planning and informed decision-making. Manufacturers are encouraged to invest in local expertise and stay updated on regulatory changes to maximize their potential for success. By prioritizing compliance and leveraging the resources available through specialized services like bioaccess®, companies can navigate the Mexican market with greater confidence and efficiency, ultimately contributing to the growth of healthcare innovation in the region.
What is bioaccess® and how does it assist with medical device registration in Mexico?
bioaccess® is a service that leverages regional expertise to expedite medical device registration in Mexico, allowing for health product approvals in as little as 4-6 weeks. This rapid process is crucial for Medtech innovators to quickly introduce their products while complying with COFEPRIS regulations.
What are COFEPRIS regulations?
COFEPRIS, the Federal Commission for Protection against Sanitary Risks, is the regulatory authority in Mexico responsible for ensuring that healthcare instruments meet safety, effectiveness, and quality standards before entering the market.
What is the significance of complying with COFEPRIS standards?
Compliance with COFEPRIS standards is essential for producers to navigate the complexities of the Mexican business landscape effectively and is crucial for ensuring the safety of healthcare products, enhancing consumer confidence, and facilitating market entry.
What are the key steps in the medical device registration process in Mexico?
The key steps include: 1. Classification of the device based on risk level (Class I, II, or III). 2. Preparation of comprehensive documentation, including technical and clinical information. 3. Submission of the application to COFEPRIS with the necessary fees. 4. Review by COFEPRIS, which may request additional information. 5. Approval and issuance of a registration certificate, allowing the product to be marketed.
How long does the registration process typically take?
The registration process usually takes 4 to 6 weeks, which is significantly quicker compared to many conventional sectors.
What services does bioaccess® offer to support medical device registration?
bioaccess® offers comprehensive clinical trial management services, including Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies, to ensure products meet COFEPRIS standards efficiently.
Why is engaging with local regulatory experts beneficial for Medtech companies?
Engaging with local regulatory experts helps Medtech companies navigate COFEPRIS regulations more effectively, streamlining the registration process and minimizing errors, ultimately enhancing the likelihood of successful market entry.
What is the market potential for medical devices in Mexico?
Mexico is the second-largest healthcare equipment market in Latin America, valued at approximately $6.25 billion in 2022, presenting a lucrative opportunity for companies that prioritize regulatory efficiency and compliance.