


The article titled "8 Key Medical Device FDA Regulations Every Innovator Must Know" serves as a critical guide for medical device innovators, outlining essential FDA regulations necessary for compliance and successful market entry. It delineates the classification of medical devices into three risk categories, underscores the importance of clinical trials for FDA approval, and stresses the necessity of ongoing education to adeptly navigate the evolving regulatory landscape. These factors are not merely procedural; they are vital for ensuring patient safety and maintaining a competitive edge within the Medtech sector.
Navigating the intricate landscape of medical device regulations is essential for innovators aiming to successfully bring their products to market. The FDA's stringent guidelines dictate everything from classification to post-market surveillance, making an understanding of these regulations not just beneficial—it's imperative.
As the medical device sector evolves rapidly, innovators must consider: how can they ensure compliance while also expediting their path to market?
This article delves into the eight key FDA regulations that every medical device innovator must know, providing insights that can significantly impact their journey in the competitive healthcare industry.
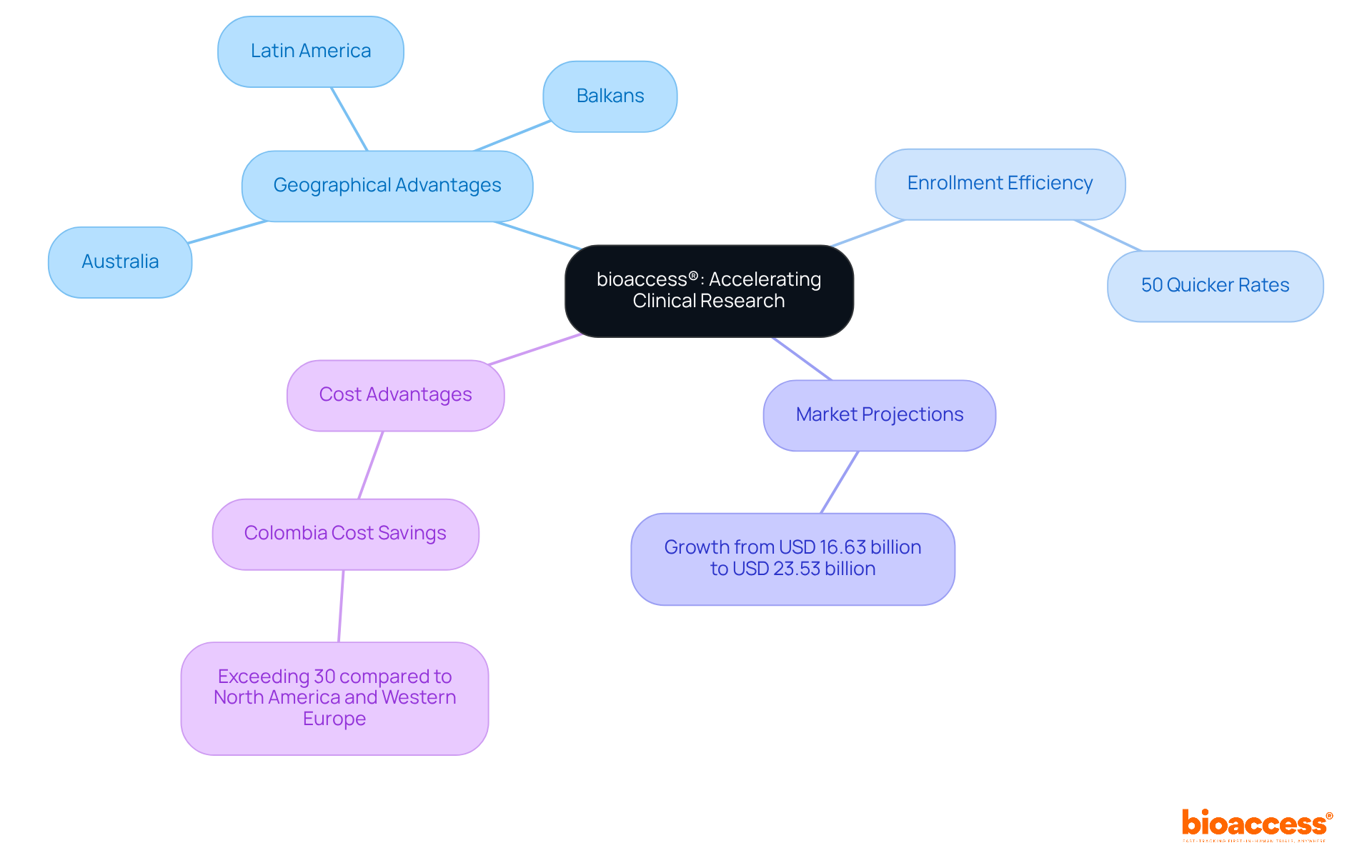
bioaccess® leverages its extensive knowledge and local advantages to significantly enhance the clinical research process for healthcare products. By capitalizing on Latin America's governance speed, the Balkans' diverse patient demographics, and Australia's efficient ethical approval pathways, bioaccess® achieves ethical approvals in an impressive 4-6 weeks. This remarkable efficiency results in patient enrollment rates that are 50% quicker than those in conventional markets, a crucial advantage for innovators aiming to expedite their healthcare products' market entry. With nearly 90% of research studies failing to meet enrollment targets, this flexibility not only aids in fulfilling medical device FDA regulations but also greatly accelerates the advancement of healthcare technology.
As the global healthcare equipment clinical trials market is projected to grow from USD 16.63 billion in 2024 to USD 23.53 billion by 2030, understanding and complying with medical device FDA regulations is vital for maintaining a competitive edge in the Medtech sector. Moreover, Colombia presents cost savings exceeding 30% compared to North America and Western Europe, making it a highly attractive location for conducting clinical trials. The country boasts a healthcare system ranked among the best in Latin America, with a population of over 50 million and 95% coverage under universal healthcare, ensuring a robust patient recruitment base. Additionally, Colombia offers significant R&D tax incentives, including a 100% tax deduction for investments in science and technology, further enhancing its appeal as a premier destination for clinical trials.
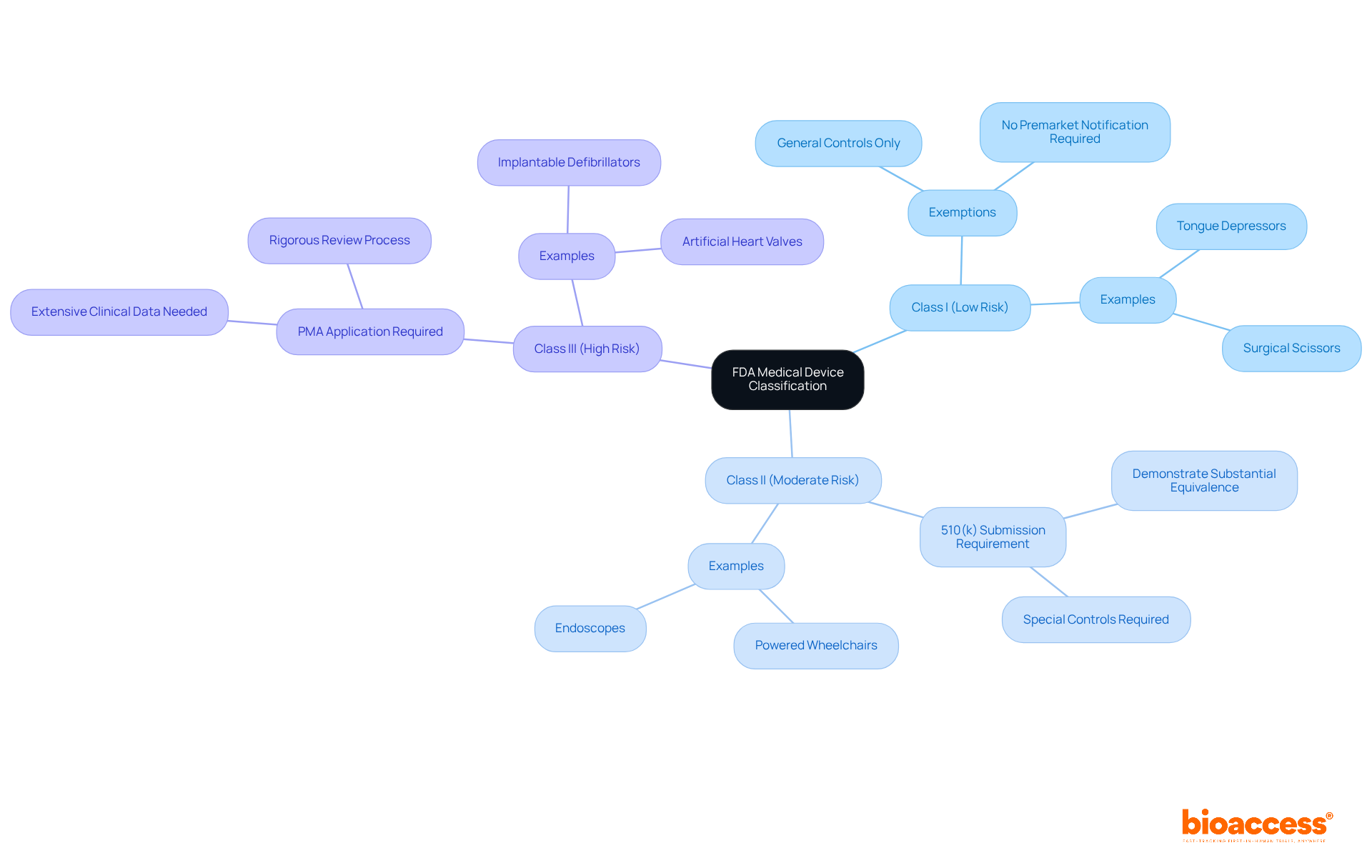
The FDA classifies healthcare instruments into three distinct categories based on risk:
Notably, around 43% of medical equipment is categorized under Class II, which includes more complex designs such as powered wheelchairs and endoscopes. Class I items, like tongue depressors and surgical scissors, are generally exempt from premarket notification, requiring only basic regulatory controls. In contrast, Class II products necessitate a 510(k) submission to demonstrate substantial equivalence to a legally marketed predicate product, ensuring compliance with specific safety and effectiveness standards.
Class III products, which encompass high-risk items such as implantable defibrillators and artificial heart valves, require a Premarket Approval (PMA) application. This rigorous process demands extensive clinical data to verify the safety and efficacy of the devices, often involving comprehensive studies and strict adherence to the FDA's Quality System Regulation (QSR). As of January 2025, the FDA is committed to responding to 95% of written Q-Submissions within 70 calendar days, thereby streamlining the approval process for innovators.
Understanding these classifications is crucial for medical device creators, as they dictate the approval process, data requirements, and the level of oversight under medical device FDA regulations. Early collaboration with compliance specialists, such as Katherine Ruiz at bioaccess®, can provide invaluable insights into the specific requirements for each class. Bioaccess® excels in managing clinical trials across various stages, including Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies. By leveraging bioaccess®'s expertise and connections with top-ranked clinical research sites, innovators can navigate the complex regulatory landscape more effectively and expedite their path to market.
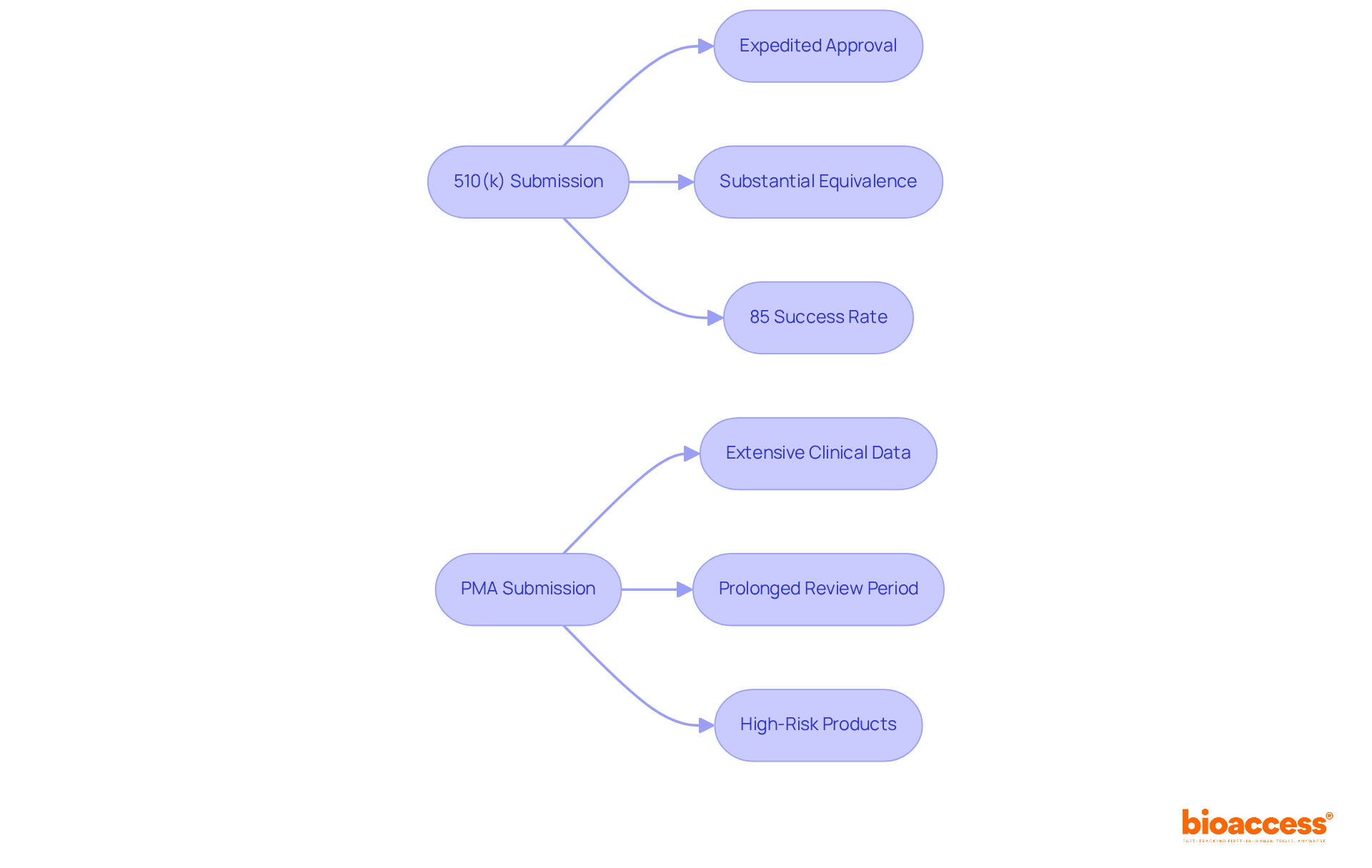
The 510(k) submission process facilitates expedited approval for instruments demonstrating substantial equivalence to existing products, presenting a more efficient and cost-effective pathway for manufacturers. Conversely, the Premarket Approval (PMA) process is markedly more demanding, requiring extensive clinical data and a prolonged review period, often exceeding a year.
Innovators must meticulously evaluate their product's risk profile and identify appropriate market equivalents to ascertain the most suitable submission route. This decision is pivotal, as it significantly influences the timeline and resources allocated for market entry.
For instance, in 2021:
Industry leaders underscore the importance of understanding the medical device FDA regulations, as the choice between 510(k) and PMA can dictate not only compliance success but also the overall market strategy for innovative healthcare technologies.
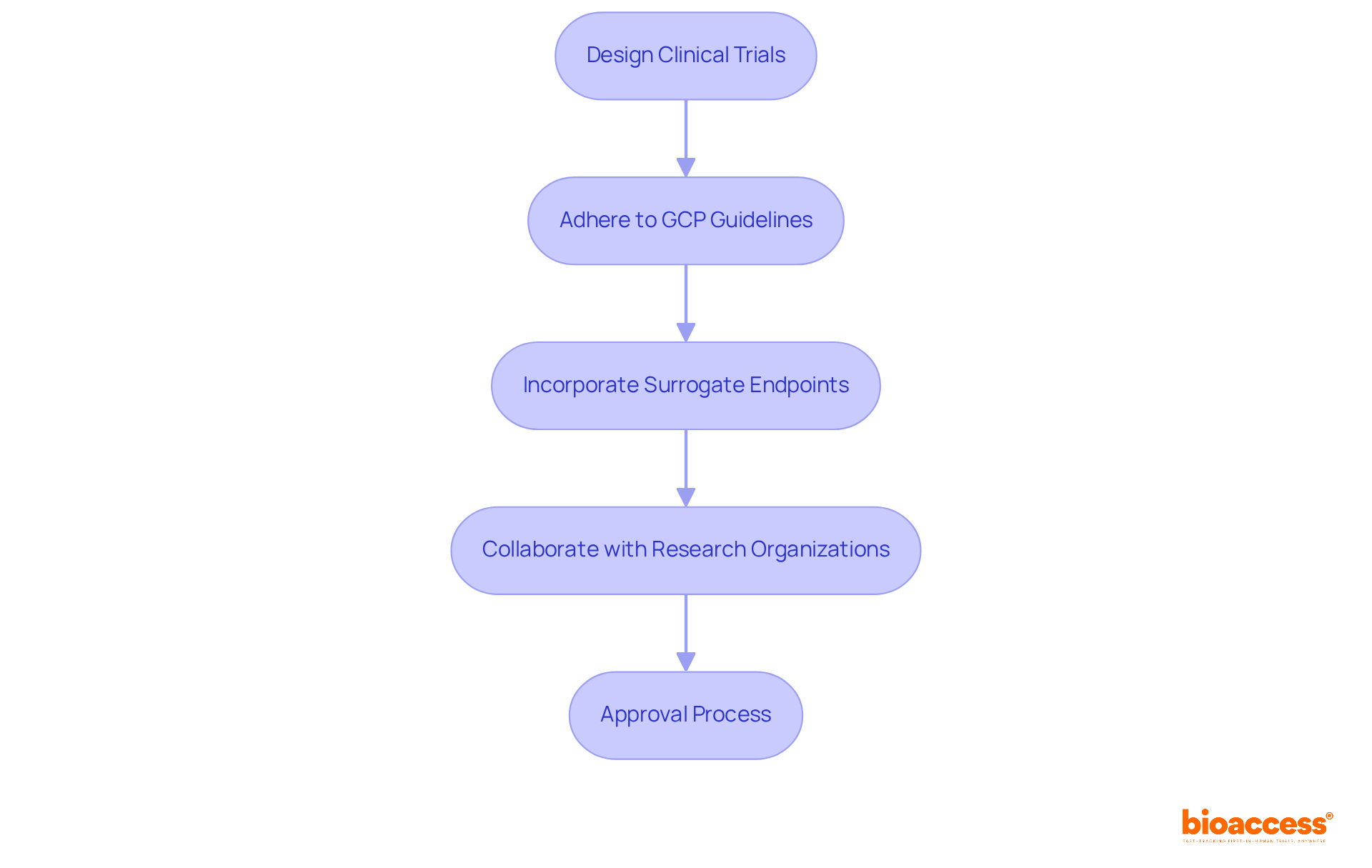
Conducting clinical trials is an essential step in securing approval for medical equipment under medical device FDA regulations. These trials must be meticulously designed to produce valid scientific evidence regarding the apparatus's safety and effectiveness. The medical device FDA regulations require strict adherence to Good Clinical Practice (GCP) guidelines, which protect participant welfare and ensure data integrity.
Successful clinical trial designs often incorporate validated surrogate endpoints to qualify for accelerated approval, particularly for serious conditions. Collaborating with experienced clinical research organizations, such as bioaccess®, can significantly simplify the complexities of trial design and execution. This partnership not only increases the likelihood of meeting regulatory expectations but also facilitates a more efficient approval process.
On average, clinical trials for FDA approval can take anywhere from 10 to 15 years, highlighting the necessity of strategic planning and execution in the trial design phase.
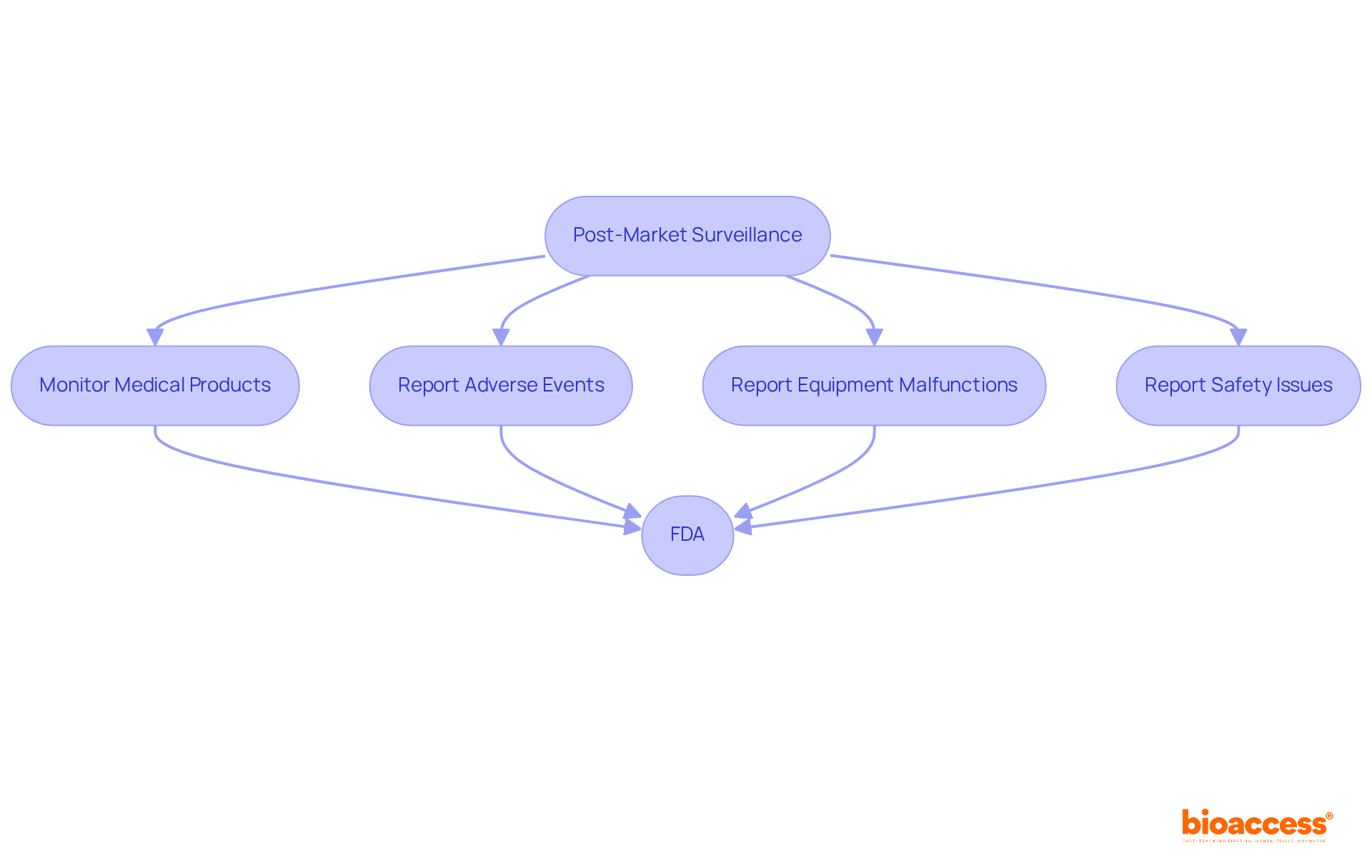
Post-market surveillance is essential for the ongoing monitoring of medical products once they are available for sale. Producers are mandated to report any adverse events, equipment malfunctions, or safety issues to the FDA, which receives approximately 200,000 case reports annually. This continuous oversight is vital for maintaining the safety and effectiveness of tools throughout their lifecycle.
To comply with medical device FDA regulations and protect patient safety, companies must establish robust post-market surveillance systems. These systems not only fulfill compliance standards but also enhance a company's reputation and foster trust in the market. Regulatory specialists emphasize that effective post-market surveillance can significantly reduce risks associated with healthcare products, ensuring that any emerging safety concerns are promptly addressed.
For example, the FDA's recent initiatives aim to streamline reporting processes and enhance the accuracy of adverse event data, underscoring the necessity of proactive compliance strategies. By developing comprehensive monitoring systems, manufacturers can more effectively navigate the complexities of medical device FDA regulations and improve the overall safety of healthcare instruments.
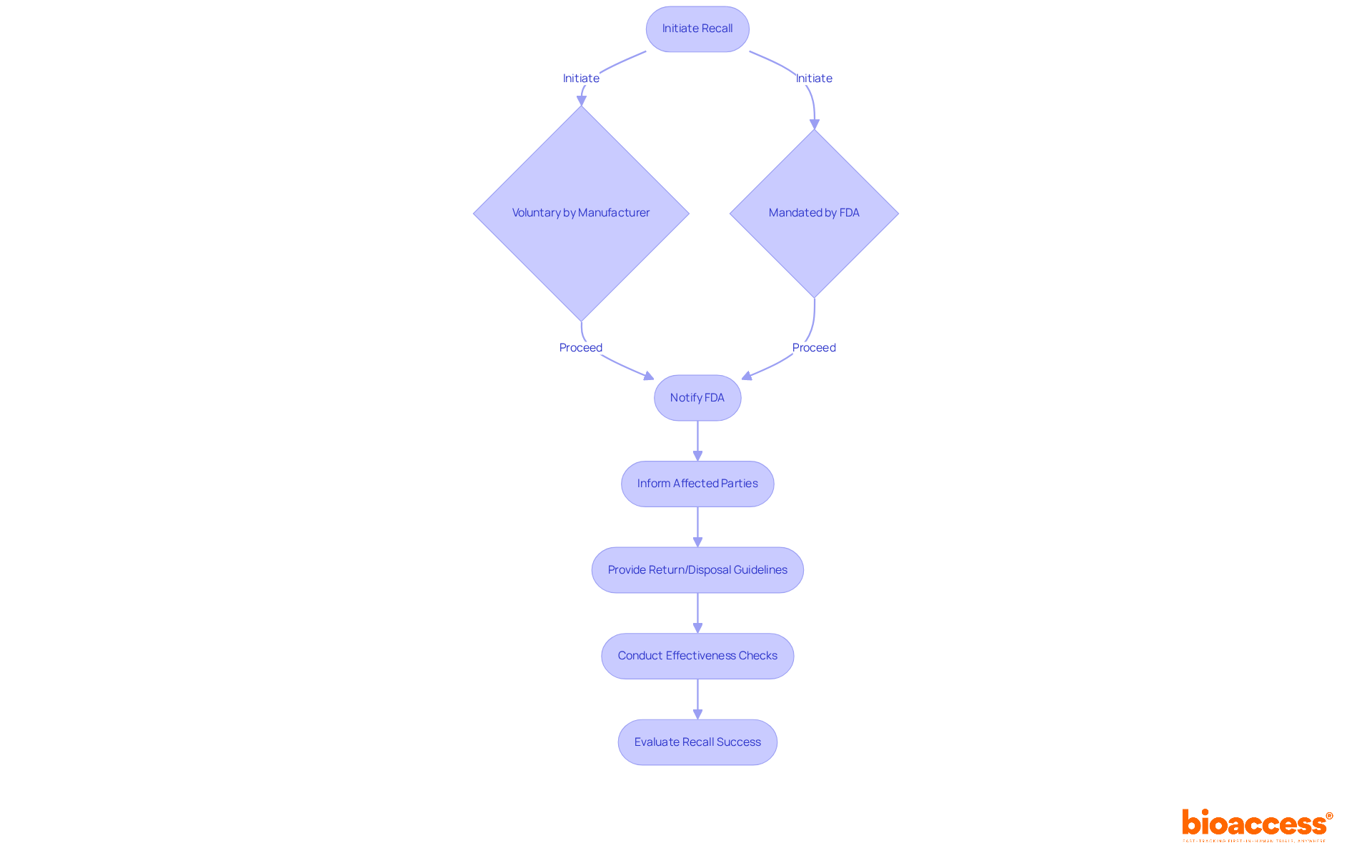
The FDA has established a comprehensive framework for recalling medical products that may present health risks. A recall can be initiated voluntarily by the manufacturer or mandated by the FDA, reflecting an increasing emphasis on patient safety and regulatory compliance. The process commences with notifying the FDA, followed by informing affected parties, including healthcare providers and consumers. Producers are required to supply clear guidelines for the return or appropriate disposal of the item, ensuring that safety measures are communicated effectively.
Furthermore, manufacturers must conduct effectiveness checks to evaluate the recall's success, which is essential for mitigating risks associated with the recalled product. Recent updates indicate that the average time for the FDA to process product recalls has improved, with many recalls being addressed within weeks, underscoring the agency's commitment to swift action. For instance, the FDA has reported that the average processing time for recalls has decreased to approximately 30 days, a significant enhancement that bolsters the responsiveness of the regulatory framework.
Insights from industry leaders underscore the importance of a well-structured recall strategy. A successful voluntary recall by Smiths Medical involved proactive communication and thorough follow-up regarding three identified issues with their infusion pumps, which helped sustain trust with healthcare professionals and the public. Such instances highlight the critical role of transparency and accountability in managing product recalls.
As of 2025, the FDA continues to refine its recall processes, ensuring that manufacturers are equipped with the necessary guidelines to navigate these situations effectively. Understanding these procedures is vital for manufacturers aiming to uphold compliance with medical device FDA regulations while prioritizing patient safety. Mishandling a recall can result in severe consequences, including legal liabilities and financial losses, further emphasizing the necessity for a comprehensive recall strategy.
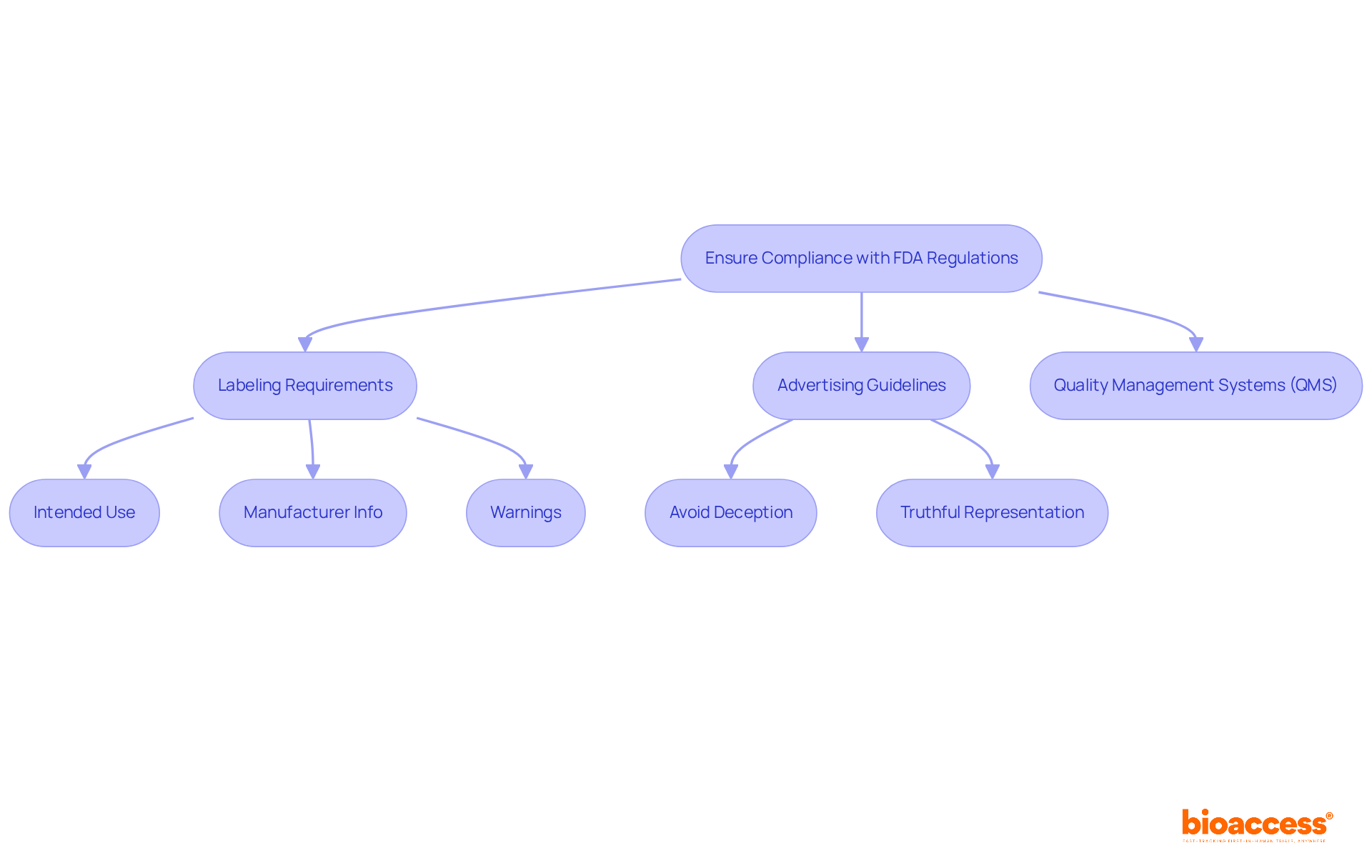
Medical device FDA regulations require that the labeling of medical equipment be not only clear and accurate but also informative. Labels must specify the product's intended use, include the manufacturer's information, and provide necessary warnings or precautions. Moreover, advertising must avoid deceptive assertions and truthfully represent the product's capabilities.
In fiscal year 2023, the FDA issued 24 Warning Letters related to labeling violations, underscoring the critical need for compliance. Effective labeling strategies frequently involve early interaction with oversight agencies and the establishment of robust quality management systems (QMS) in compliance with medical device FDA regulations to ensure that all documentation meets standards.
Regulatory experts stress that viewing compliance as an ongoing commitment to patient safety and product quality is essential. Staying updated on advertising regulations is crucial, particularly with the evolving landscape in 2025, where new guidelines may emerge. By adhering to these requirements, companies can ensure that consumers are well-informed, thereby minimizing the risk of legal issues and enhancing trust in their products.
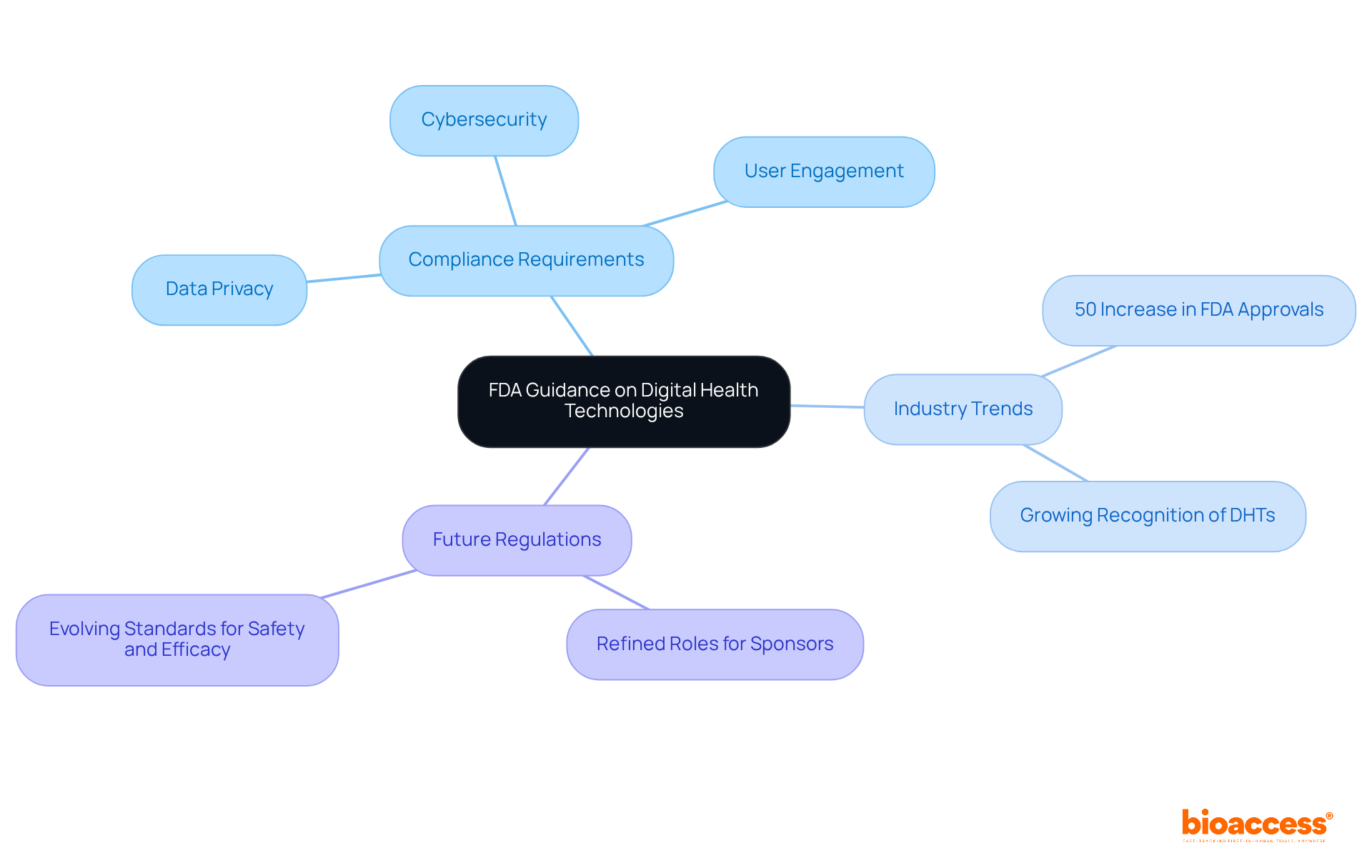
The FDA has released updated guidance on the regulation of digital health technologies (DHTs), emphasizing the critical importance of transparency and safety in the development of software and devices. Innovators must navigate specific compliance requirements, which include:
As the digital health landscape evolves, staying abreast of FDA guidance is vital for ensuring compliance and successfully introducing innovative solutions to the market. Notably, specialists such as Ana Criado, with her extensive experience in compliance matters and biomedical engineering, stress the necessity of aligning product development with industry expectations.
Recent trends indicate a significant increase in FDA approvals for digital health products, marked by a 50% rise in 2024 compared to the previous year. This surge reflects the growing recognition of DHTs' potential to enhance patient care and streamline clinical trials. Innovators are urged to adopt a proactive stance towards compliance, leveraging insights from industry leaders who emphasize the importance of aligning product development with legal expectations.
Looking ahead to 2025, updates to regulations will likely refine the roles of sponsors and investigators in DHT utilization, ensuring that all digital health products meet the evolving standards for safety and efficacy. By prioritizing these compliance aspects, innovators can better position themselves to navigate the complexities of the governance landscape and capitalize on the burgeoning opportunities within the digital health sector.
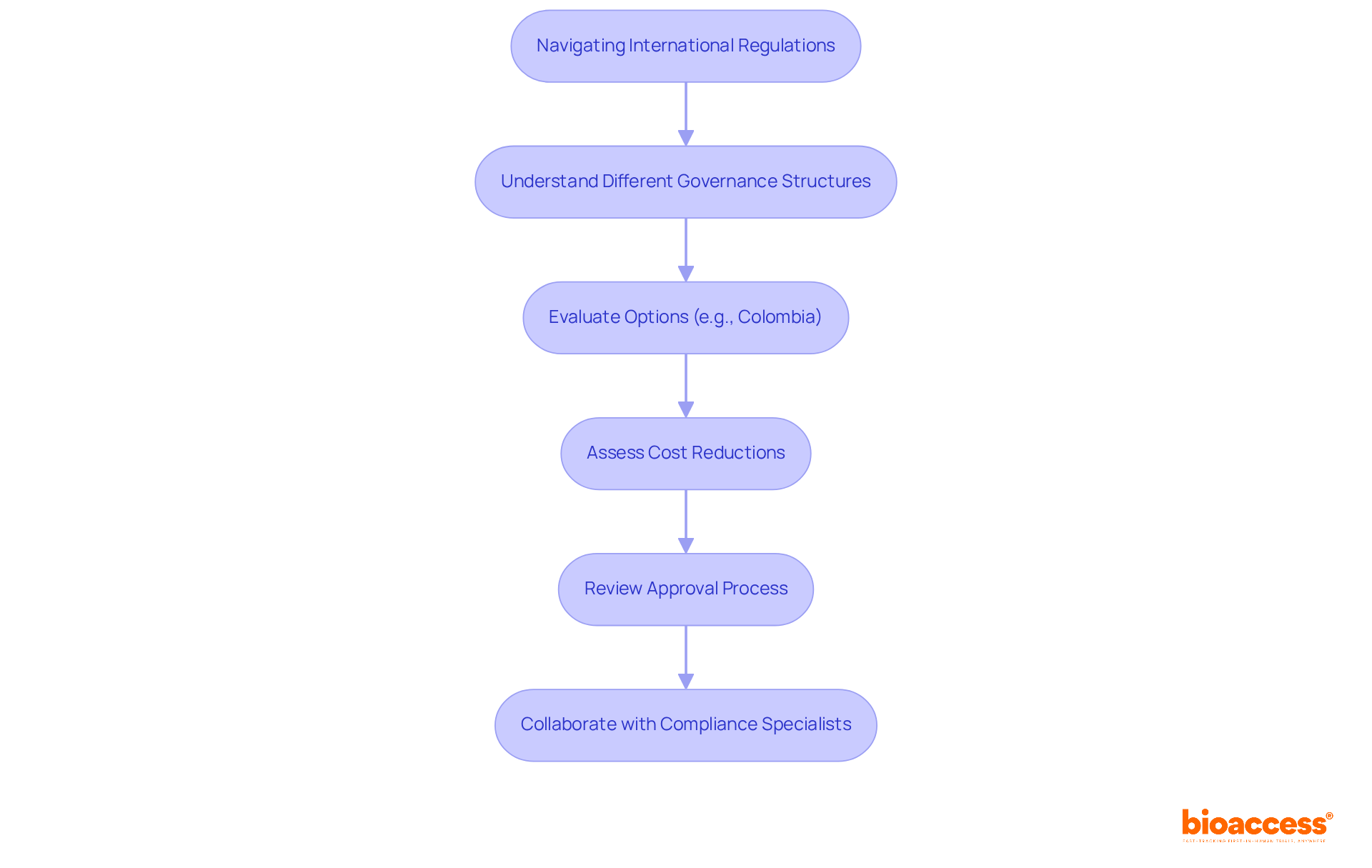
Innovators must navigate a complex landscape of international regulations alongside the medical device FDA regulations. Each nation possesses its own governance structure, which can differ significantly from the U.S. system. For instance, Colombia presents an attractive option for healthcare equipment trials, offering substantial cost reductions exceeding 30% compared to North America and Western Europe. Additionally, the efficient approval process generally requires only 90-120 days for IRB/EC and INVIMA authorizations. Understanding these differences is essential for successful market entry in global markets.
Collaborating with compliance specialists and organizations like bioaccess™ can simplify this process, ensuring adherence to both local and international guidelines while leveraging Colombia's high-quality healthcare system and robust patient recruitment capabilities. This strategic partnership can markedly enhance the efficiency of clinical trials, facilitating innovators in bringing their products to market.
The oversight environment for healthcare instruments is undergoing continuous change, underscoring the essential need for ongoing education among producers and innovators. In 2025, customized training programs aimed at compliance updates related to healthcare equipment—such as workshops on FDA regulations and online certification courses—are crucial for staying abreast of evolving standards.
Engaging in specialized training programs, attending industry conferences, and subscribing to compliance updates are effective strategies for stakeholders to remain informed about significant changes and best practices. Insights from industry leaders, including Ana Criado, Director of Regulatory Affairs and CEO of Mahu Pharma, highlight that proactive education not only bolsters compliance efforts but also empowers companies to adeptly navigate the complexities of the medical device market.
Criado's extensive background in biomedical engineering and health economics, coupled with her experience as a compliance consultant for global firms, underscores the importance of comprehensive training programs that focus on the latest compliance trends. By ensuring their teams are well-versed in compliance requirements, manufacturers can maintain a competitive edge and uphold the highest standards of safety and efficacy as regulatory updates continue to shape the industry.

Understanding the intricate landscape of FDA regulations for medical devices is essential for innovators aiming to bring their products to market successfully. This article has underscored the importance of navigating various regulatory pathways, from classification and approval processes to post-market surveillance and recall procedures. By grasping these key regulations, innovators can ensure compliance, enhance patient safety, and streamline their product development timelines.
Throughout the discussion, several crucial points emerged:
Additionally, ongoing education and awareness of international regulations play a vital role in maintaining a competitive edge in the rapidly evolving Medtech sector. Collaborating with experienced organizations like bioaccess® can further facilitate a smoother regulatory journey, ultimately accelerating the introduction of innovative healthcare solutions.
As the landscape of medical device regulation continues to evolve, staying informed and proactive is imperative. Innovators are encouraged to engage in continuous education, leverage available resources, and embrace compliance as a cornerstone of their strategy. By doing so, they not only safeguard their products but also contribute to the advancement of healthcare technologies that can significantly improve patient outcomes.
What is bioaccess® and how does it enhance the clinical research process for medical devices?
Bioaccess® leverages its extensive knowledge and local advantages to significantly enhance the clinical research process by achieving ethical approvals in 4-6 weeks, resulting in patient enrollment rates that are 50% quicker than conventional markets.
Why is the speed of ethical approvals important for medical device innovators?
The speed of ethical approvals is crucial as it helps innovators expedite their healthcare products' market entry, especially since nearly 90% of research studies fail to meet enrollment targets.
What are the projected growth figures for the global healthcare equipment clinical trials market?
The global healthcare equipment clinical trials market is projected to grow from USD 16.63 billion in 2024 to USD 23.53 billion by 2030.
What advantages does Colombia offer for conducting clinical trials?
Colombia offers cost savings exceeding 30% compared to North America and Western Europe, a robust healthcare system, a population of over 50 million with 95% universal healthcare coverage, and significant R&D tax incentives, including a 100% tax deduction for investments in science and technology.
How does the FDA classify medical devices?
The FDA classifies medical devices into three categories based on risk: Class I (low risk), Class II (moderate risk), and Class III (high risk).
What is the submission process for Class II and Class III medical devices?
Class II devices require a 510(k) submission to demonstrate substantial equivalence to a legally marketed product, while Class III devices require a Premarket Approval (PMA) application, which necessitates extensive clinical data to verify safety and efficacy.
What is the significance of the FDA's commitment to respond to Q-Submissions?
As of January 2025, the FDA aims to respond to 95% of written Q-Submissions within 70 calendar days, streamlining the approval process for innovators.
What is the difference between the 510(k) submission process and the PMA process?
The 510(k) submission process is more efficient and cost-effective, requiring evidence of substantial equivalence to existing products, while the PMA process is more demanding, requiring extensive clinical data and typically taking over a year for review.
How can innovators determine the appropriate submission route for their medical device?
Innovators must evaluate their product's risk profile and identify appropriate market equivalents to decide between the 510(k) and PMA submission routes, as this choice significantly influences the timeline and resources for market entry.
Why is understanding FDA medical device regulations important for innovators?
Understanding FDA regulations is vital as it dictates the approval process, data requirements, and oversight level, influencing compliance success and overall market strategy for innovative healthcare technologies.