


The article delineates the device market clearance requirements in Mexico for medical devices, outlining the essential steps and documentation that manufacturers must follow to achieve successful approval. It underscores the critical nature of compliance with local regulations, which encompasses:
These elements collectively facilitate a more streamlined approval process, thereby ensuring both the safety and efficacy of medical products within the expanding healthcare market.
Navigating the medical device market in Mexico presents both opportunities and challenges for manufacturers aiming to introduce innovative healthcare solutions.
With a rapidly evolving regulatory landscape overseen by COFEPRIS, understanding the device market clearance requirements is crucial for success.
This article delves into the essential steps and documentation necessary for compliance, highlighting how companies like bioaccess® can streamline the approval process and enhance market entry efficiency.
As the demand for advanced medical technologies grows, the question remains: how can manufacturers effectively navigate these complex regulations to ensure timely access to this burgeoning market?
bioaccess® excels in expediting the device market clearance requirements in Mexico by leveraging its extensive knowledge of local regulations and accelerating ethical approvals. This strategic approach not only guarantees compliance but also significantly reduces time to market, empowering innovators to capitalize on Mexico's burgeoning healthcare sector.
With ethical approvals achieved in just 4-6 weeks and enrollment rates 50% faster than traditional sectors, bioaccess® positions itself as an indispensable ally for device firms navigating the device market clearance requirements in Mexico. Industry leaders recognize the critical need to expedite oversight procedures, particularly as the growing elderly population and the increasing prevalence of chronic diseases drive demand for innovative healthcare solutions.
Furthermore, bioaccess® activates over 50 sites in under 8 weeks, delivering FDA/EMA/MDR-ready datasets with centralized monitoring. As the Mexican MedTech landscape evolves, bioaccess® remains committed to facilitating swift market entry for its clients by helping them navigate the device market clearance requirements in Mexico, ensuring they can meet the rising demand for advanced healthcare technologies while saving $25K per patient with FDA-ready data—no rework, no delays.
COFEPRIS, the Federal Commission for Protection against Sanitary Risks, serves as Mexico's primary authority for healthcare instruments, ensuring their safety and effectiveness. As of July 2025, COFEPRIS has streamlined its guidelines for equipment registration, reflecting a commitment to enhancing the oversight environment. Manufacturers seeking approval must navigate a structured process that entails:
Over the past year, COFEPRIS has documented a significant volume of healthcare instruments, underscoring the growing demand for innovative health solutions in the country.
Successful interactions with COFEPRIS depend on a thorough understanding of its requirements and the maintenance of open communication. Regulatory experts underscore the necessity of meticulous preparation, including the submission of all requisite documents to comply with the device market clearance requirements in Mexico. This proactive strategy not only facilitates smoother approvals but also nurtures a collaborative relationship with the regulatory body. Manufacturers are urged to remain informed about ongoing updates and changes in COFEPRIS guidelines to ensure compliance and expedite the approval process.
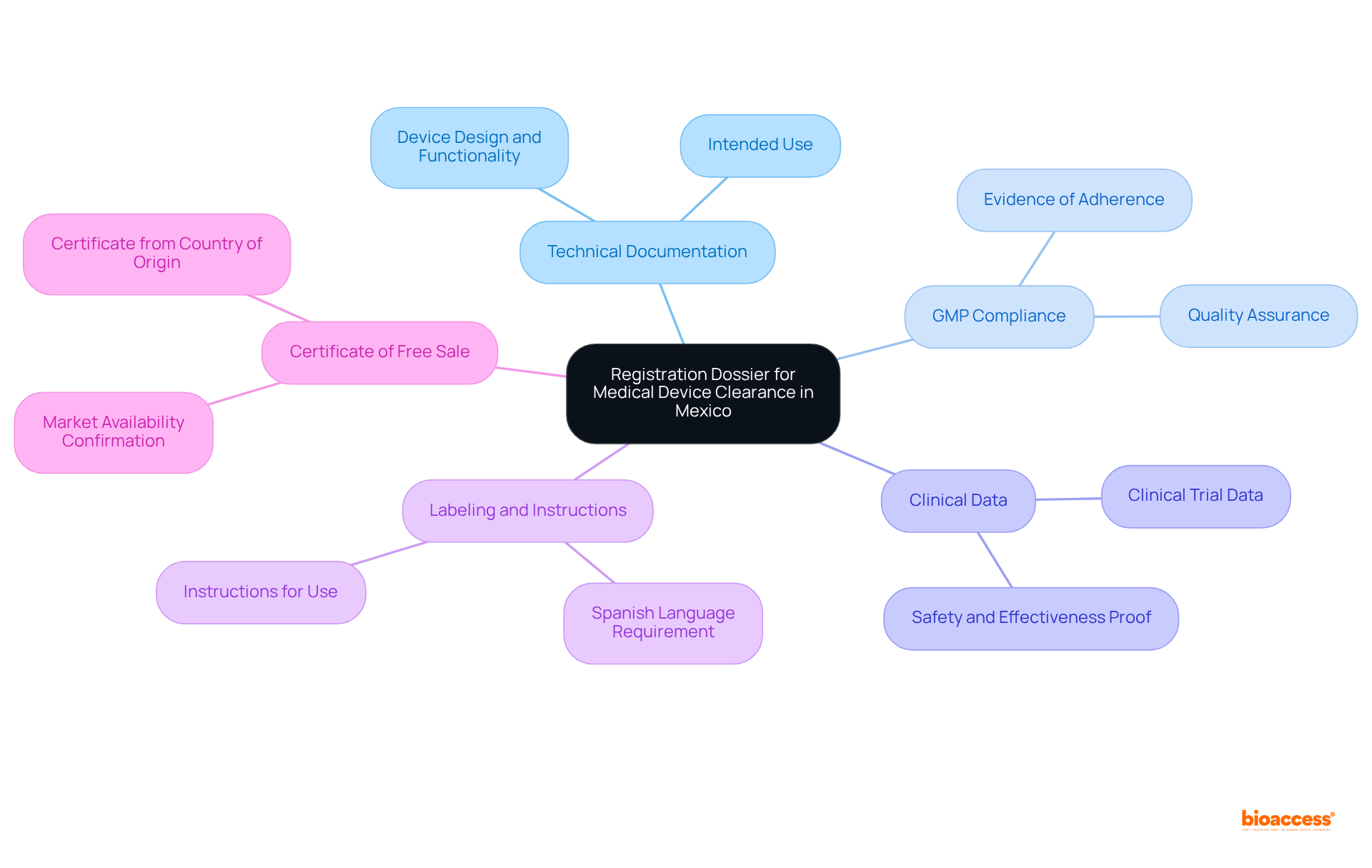
To secure market clearance for medical devices in Mexico, manufacturers must compile a detailed registration dossier that encompasses several essential components:
These documents are submitted to COFEPRIS for thorough review and approval. It's important to note that common documentation errors can lead to delays; for instance, 32% of FDA 510(k) submissions failed the minimum acceptability check in 2022, highlighting the need for meticulous preparation. By ensuring that all components of the registration dossier comply with the device market clearance requirements in Mexico, manufacturers can significantly enhance their chances of a successful and timely approval process.
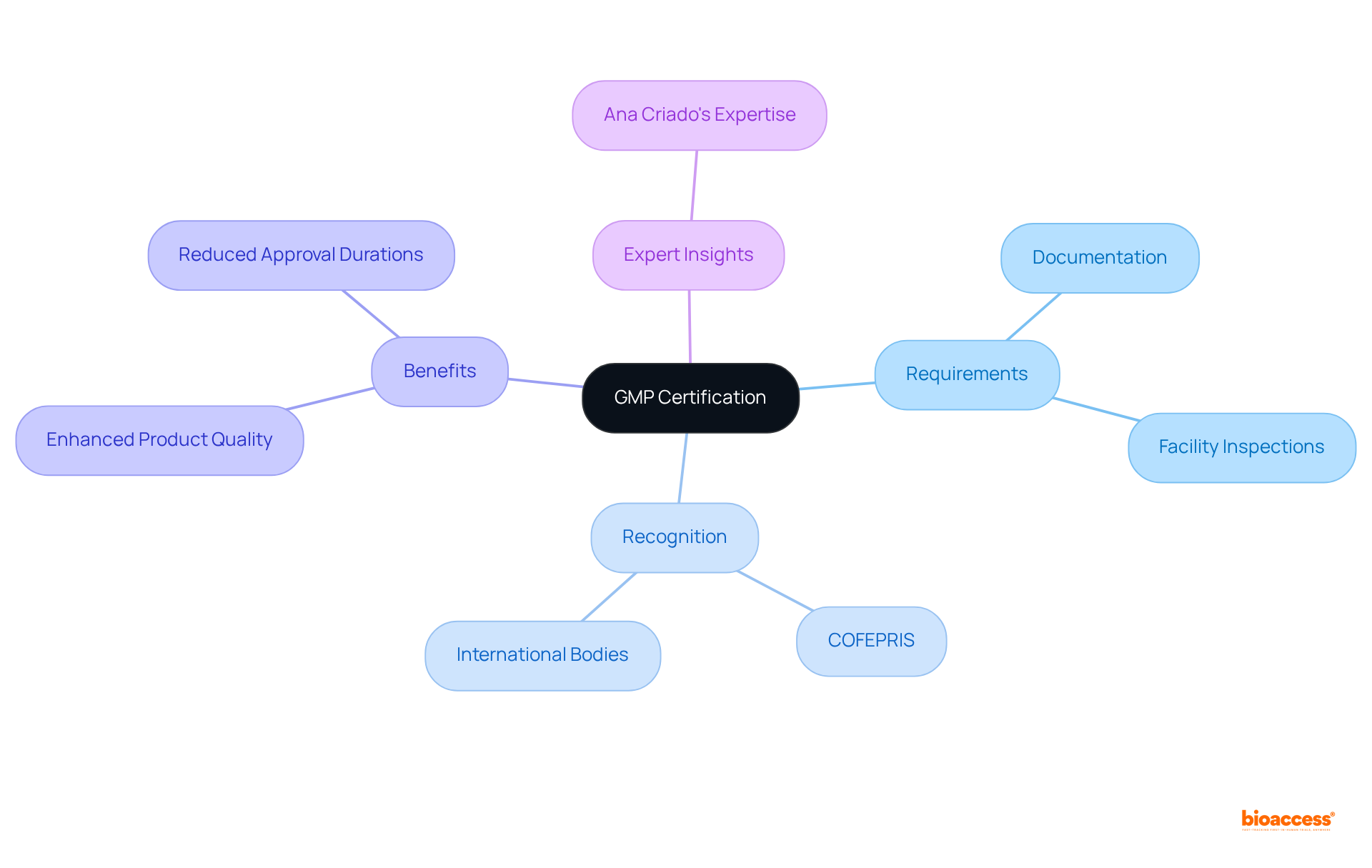
Good Manufacturing Practices (GMP) certification is essential for all health product manufacturers to comply with device market clearance requirements in Mexico. This certification guarantees that products are consistently produced and controlled according to established quality standards.
To achieve GMP compliance, manufacturers must:
The Federal Commission for the Protection against Sanitary Risk (COFEPRIS) recognizes GMP certifications from various international regulatory bodies, significantly streamlining the approval process for foreign manufacturers.
Adhering to GMP not only enhances product quality but also reduces approval durations, thus enabling quicker entry for innovative healthcare instruments that meet device market clearance requirements in Mexico. As the Mexican healthcare equipment market continues to expand, compliance with device market clearance requirements in Mexico and GMP standards becomes increasingly vital for producers aiming to thrive in this competitive landscape.
With specialists such as Ana Criado, who possesses extensive expertise in compliance matters and biomedical engineering, the focus on adherence and quality assurance in the healthcare equipment sector is more critical than ever. Her insights into the regulatory landscape highlight the significance of GMP compliance in maintaining a competitive edge.
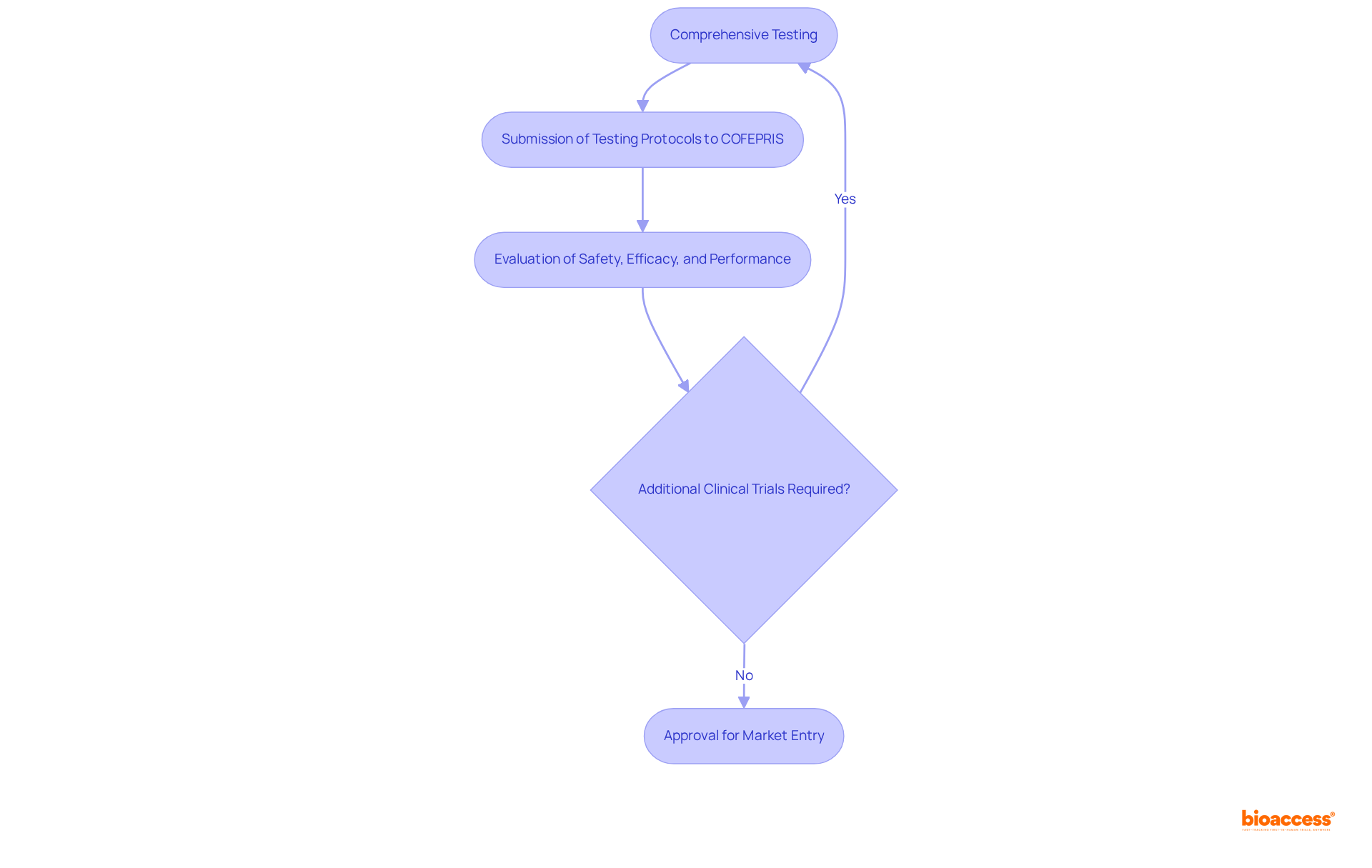
In Mexico, the device market clearance requirements for the authorization of a medical product for market entry hinge on comprehensive testing and, when necessary, clinical trials. These evaluations are essential for assessing the apparatus's safety, efficacy, and performance in real-world scenarios. Manufacturers must submit detailed testing protocols and results to the Federal Commission for Protection against Sanitary Risks (COFEPRIS) to comply with the device market clearance requirements in Mexico. Depending on the classification and intended use of the equipment, COFEPRIS may require additional clinical data to comply with the device market clearance requirements in Mexico and ensure a thorough evaluation.
The oversight environment has seen significant improvements, with COFEPRIS reducing the average setup time for clinical trials to approximately two months. This development positions Mexico as an increasingly attractive location for conducting clinical research. Such efficiency is complemented by a diverse patient pool, which enhances recruitment and accelerates the overall trial process.
As industry leaders indicate, thorough testing transcends mere compliance; it is a crucial element in ensuring that medical products meet the highest standards of safety and effectiveness prior to market entry. This commitment to rigorous evaluation not only fosters trust among stakeholders but also underscores the importance of collaboration in advancing clinical research.
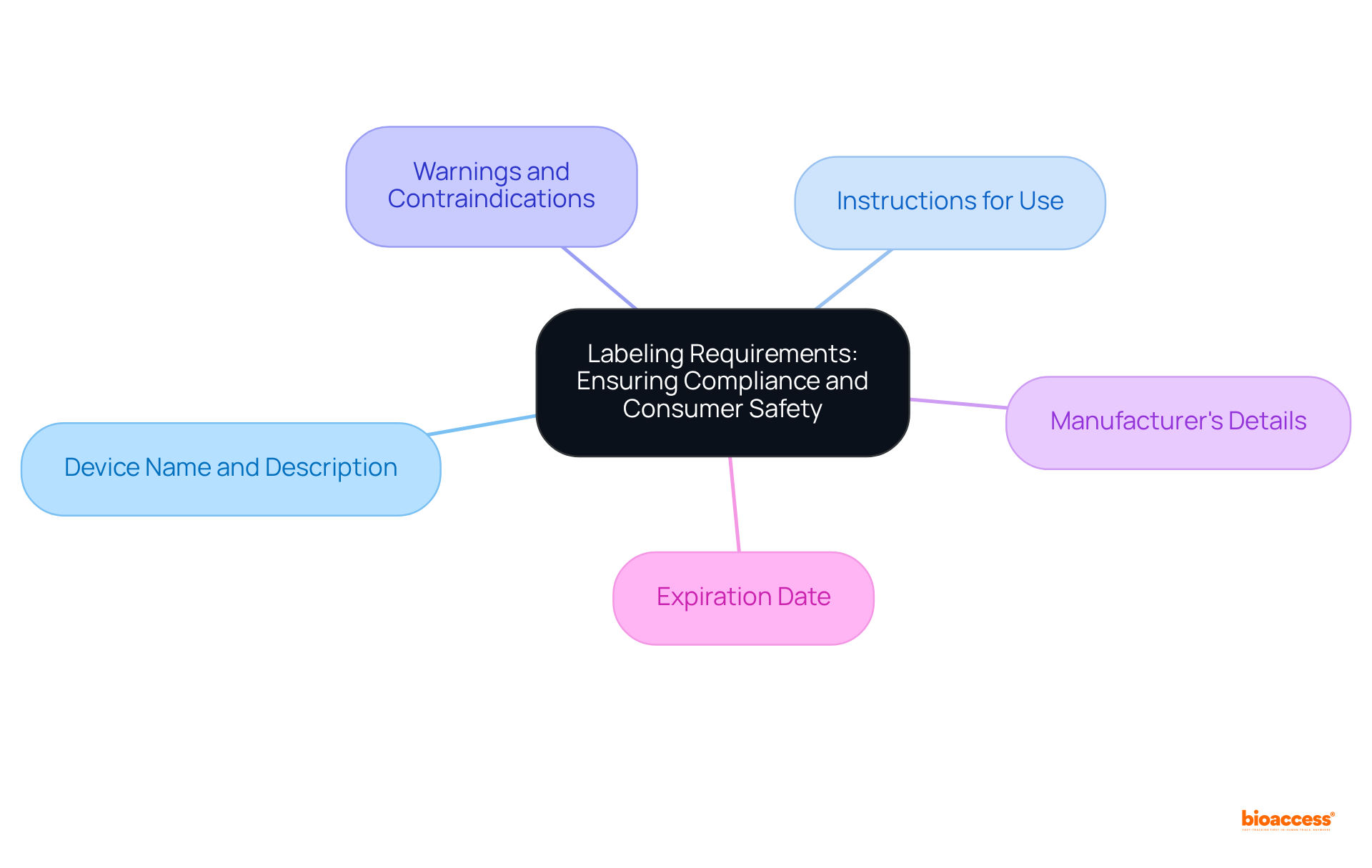
Medical equipment promoted in Mexico must adhere to the device market clearance requirements Mexico, which include strict labeling standards set by COFEPRIS, a crucial aspect for ensuring successful market entry. Labels are required to be in Spanish and must include the following essential information:
Non-compliance with these labeling standards can lead to significant delays or outright rejections in the approval process. This underscores the necessity for meticulous attention to detail in submissions. Common labeling errors include omissions of required information or inaccuracies in translations, which can jeopardize the approval timeline. To navigate these complexities effectively, it is advisable to consult with compliance experts who can provide insights into successful labeling strategies and ensure adherence to the device market clearance requirements in Mexico as well as COFEPRIS guidelines.
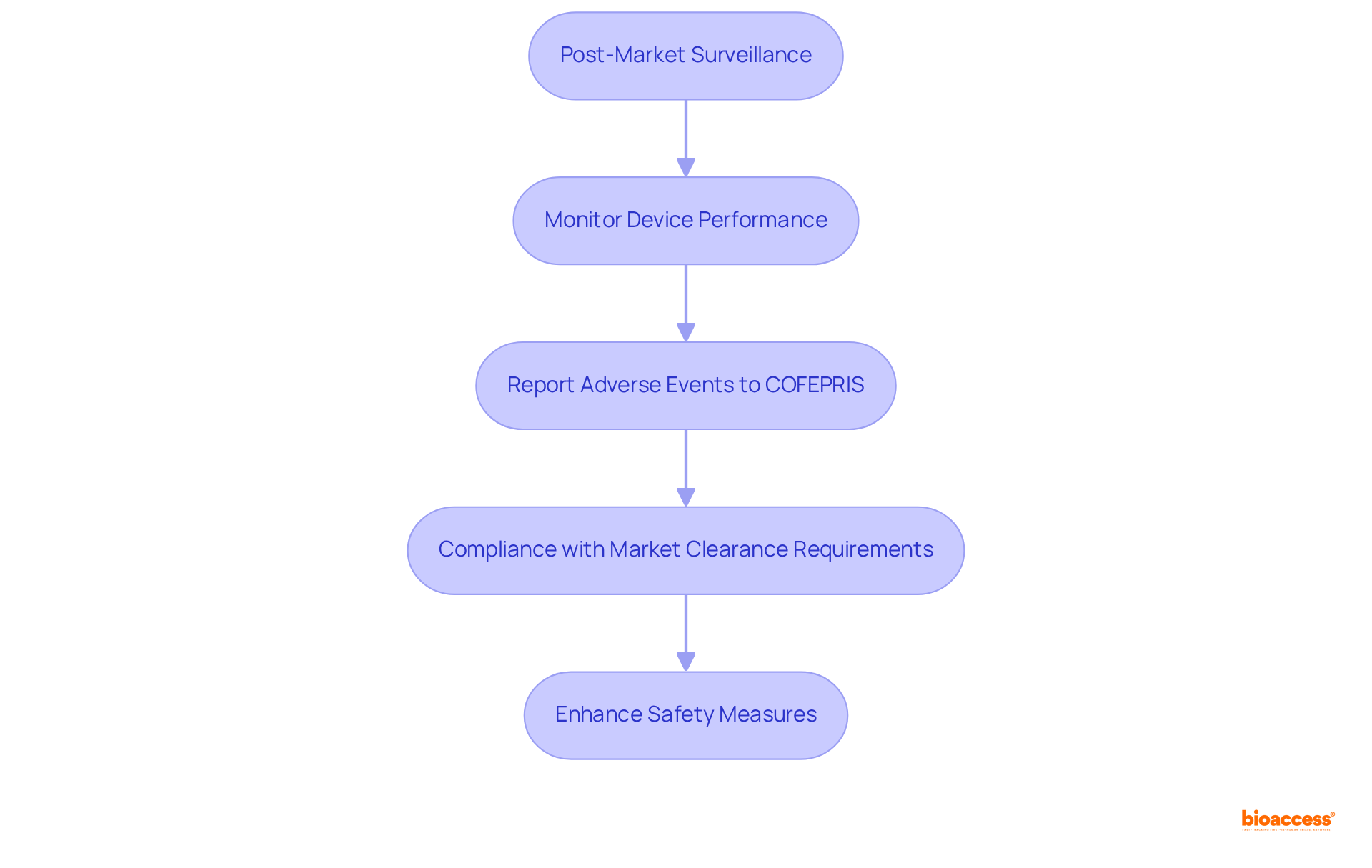
Post-market monitoring is a pivotal element in the healthcare equipment lifecycle in Mexico, where producers must adhere to device market clearance requirements by rigorously assessing the performance and safety of their products post-approval. This responsibility includes informing COFEPRIS, the regulatory body overseeing health-related instruments, about any adverse occurrences or product defects.
In recent years, an alarming total of 454,383 device-associated adverse events have been reported, underscoring the urgent need for effective monitoring systems. Industry leaders emphasize that ongoing compliance is not merely a legal requirement; it is a commitment to patient safety and the integrity of equipment. As articulated by a leading industry expert, 'Prompt adverse event reporting is a pledge to patient safety and equipment integrity.'
Moreover, COFEPRIS is actively refining its post-market surveillance processes to ensure that manufacturers comply with the device market clearance requirements and adhere to rigorous monitoring protocols. The latest updates from COFEPRIS indicate a proactive strategy aimed at enhancing safety measures, which is vital for maintaining consumer trust and ensuring the efficacy of available medical devices.
Establishing a robust post-market surveillance system is essential for safeguarding consumer safety and ensuring that any potential risks are addressed promptly and effectively. Companies like bioaccess® are instrumental in navigating these complexities, especially in addressing the device market clearance requirements, while providing expertise in clinical trials and regulatory affairs. Their extensive experience in managing various studies, including Early-Feasibility Studies (EFS) and Post-Market Clinical Follow-Up Studies (PMCF), equips manufacturers with the essential tools to uphold compliance and elevate product safety within the Mexican market.
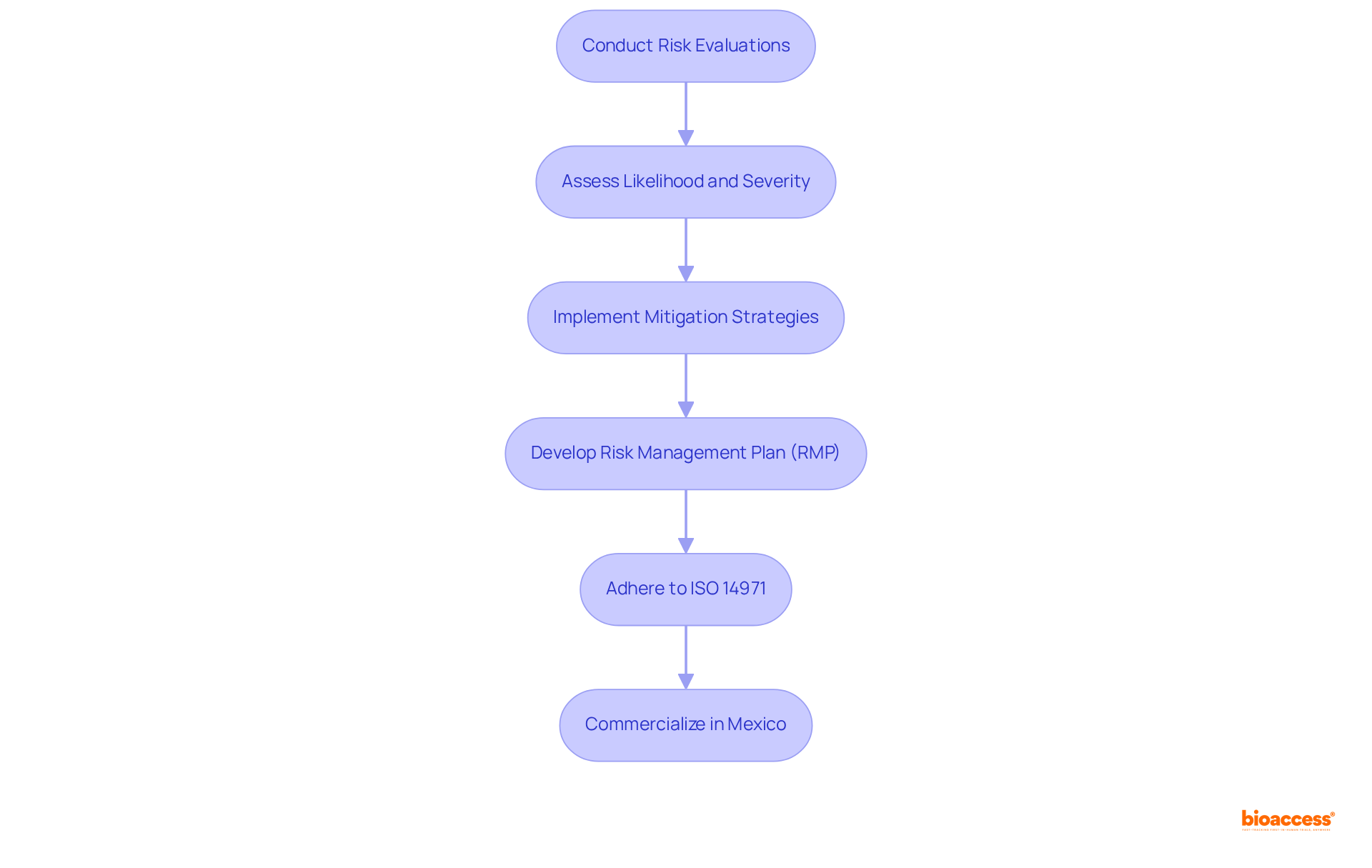
Risk management plays a pivotal role in meeting the device market clearance requirements in Mexico for healthcare products. Producers are required to conduct comprehensive risk evaluations to identify potential dangers associated with their products. This evaluation encompasses both the likelihood and severity of risks, followed by the implementation of effective mitigation strategies.
Compliance with ISO 14971, the international standard for risk management in medical devices, is a critical requirement established by COFEPRIS under the device market clearance requirements in Mexico. Recent updates in Mexico's regulatory framework highlight the necessity of developing a robust risk management plan (RMP) that includes criteria for risk acceptability in alignment with the manufacturer's policies, taking into account the device market clearance requirements in Mexico.
Efficient risk management not only enhances product safety but also fosters stakeholder trust, ultimately streamlining entry into the industry. Manufacturers are encouraged to embrace effective risk assessment strategies, such as:
to systematically identify and manage risks throughout the product lifecycle. Integrating adherence to ISO 14971 into the risk management framework is essential for ensuring that healthcare products meet safety and efficacy standards, which is necessary for fulfilling device market clearance requirements in Mexico and facilitating successful commercialization within its multi-billion dollar healthcare sector.
Furthermore, a thorough understanding of local market dynamics and regulatory nuances is crucial for navigating the complexities of the Latin American Medtech landscape, ensuring that manufacturers are well-prepared to tackle the unique challenges and opportunities that arise in this region.
International standards are pivotal in meeting the device market clearance requirements in Mexico for medical equipment. Compliance with standards such as:
is often necessary to fulfill the device market clearance requirements in Mexico. These standards not only facilitate smoother interactions with COFEPRIS but also enhance the credibility of manufacturers on the international stage.
Furthermore, effective clinical trial management services—including:
are essential for successfully navigating the compliance landscape. Experts like Ana Criado, Director of Compliance Affairs at bioaccess, offer invaluable experience in compliance matters, ensuring that medical devices adhere to necessary standards while addressing the complexities of the Colombian market, particularly in health economics and cannabis legislation.
Navigating the legal landscape in Mexico requires a thorough understanding of regional variations in clearance requirements. Each state may impose specific regulations or additional compliance obligations that manufacturers must comply with. For example, the approval process can differ significantly; some regions demand more extensive documentation or additional local approvals.
Engaging with local experts and regulatory consultants is essential, as they offer valuable insights into these nuances and can streamline the approval process. This proactive approach not only facilitates compliance but also increases the likelihood of successful market entry in a landscape marked by evolving regulations and diverse requirements.
The path to market clearance for medical devices in Mexico is intricate yet essential for manufacturers aiming to thrive in a rapidly evolving healthcare landscape. Understanding and navigating the device market clearance requirements are paramount for ensuring compliance and expediting the approval process. This ultimately allows for the timely introduction of innovative healthcare solutions to meet the needs of a growing patient population.
Throughout this article, key aspects such as the role of COFEPRIS, the importance of Good Manufacturing Practices (GMP), and the necessity of thorough documentation have been highlighted as critical components of the clearance process. Furthermore, the significance of rigorous testing, clinical trials, and post-market surveillance cannot be overstated, as they collectively ensure the safety and efficacy of medical devices. Manufacturers are encouraged to remain proactive in their compliance efforts, leveraging the expertise of specialists and staying informed about regulatory updates to navigate the complexities of the Mexican market.
In conclusion, the medical device market in Mexico presents both challenges and opportunities. By adhering to established guidelines and embracing a culture of compliance, manufacturers can enhance patient safety and position themselves competitively within the healthcare sector. Engaging with local regulatory experts and investing in robust risk management strategies will be crucial for successfully navigating the device market clearance requirements in Mexico. As the demand for advanced medical technologies continues to rise, a commitment to excellence in compliance will pave the way for sustainable growth and innovation in this vital industry.
What is bioaccess® and how does it assist with medical device market clearance in Mexico?
bioaccess® specializes in expediting medical device market clearance in Mexico by leveraging its knowledge of local regulations and accelerating ethical approvals. This approach ensures compliance and significantly reduces time to market for innovators in the healthcare sector.
How quickly can ethical approvals be achieved through bioaccess®?
Ethical approvals can be achieved in just 4-6 weeks when utilizing bioaccess®'s services.
What advantages does bioaccess® provide in terms of enrollment rates?
bioaccess® boasts enrollment rates that are 50% faster than traditional sectors, enhancing the efficiency of the approval process.
How does bioaccess® support clients in navigating the medical device market in Mexico?
bioaccess® activates over 50 sites in under 8 weeks and delivers FDA/EMA/MDR-ready datasets with centralized monitoring, helping clients meet the rising demand for advanced healthcare technologies.
Who is COFEPRIS and what role does it play in medical device regulation in Mexico?
COFEPRIS, the Federal Commission for Protection against Sanitary Risks, is Mexico's primary regulatory authority for healthcare instruments, ensuring their safety and effectiveness.
What is the process for manufacturers to obtain approval from COFEPRIS?
Manufacturers must submit comprehensive documentation, adhere to device market clearance requirements, and comply with national health standards to obtain approval from COFEPRIS.
What key components must be included in the registration dossier for medical device clearance in Mexico?
The registration dossier must include technical documentation, evidence of Good Manufacturing Practices (GMP) compliance, clinical data, labeling and instructions in Spanish, and, if applicable, a certificate of free sale from the country of origin.
Why is meticulous preparation important when interacting with COFEPRIS?
Thorough preparation, including the submission of all required documents, facilitates smoother approvals and fosters a collaborative relationship with COFEPRIS, helping to expedite the approval process.
What common issues can arise during the documentation submission process?
Common documentation errors can lead to delays in approval; for example, 32% of FDA 510(k) submissions failed the minimum acceptability check in 2022, emphasizing the importance of meticulous preparation.