


This article outlines the essential rules and best practices for effectively registering Class II medical devices with COFEPRIS in Mexico. It underscores the critical role of comprehensive documentation and adherence to classification criteria. Furthermore, the utilization of third-party reviewers is emphasized as a strategy to expedite the approval process. By doing so, manufacturers can significantly enhance compliance and market readiness, ensuring a smoother entry into the competitive Medtech landscape.
Navigating the complex landscape of medical device regulations presents a formidable challenge, particularly in Mexico, where the COFEPRIS Class II device rules impose stringent requirements. Manufacturers seeking to introduce their innovative products to this burgeoning market must not only grasp these regulations but also implement effective strategies to ensure compliance and expedite approval.
What are the key steps and best practices that can significantly enhance the likelihood of successful registration and timely market entry? This article explores the essential rules and strategies for navigating COFEPRIS Class II device regulations, providing a comprehensive roadmap for Medtech innovators eager to thrive in this competitive environment.
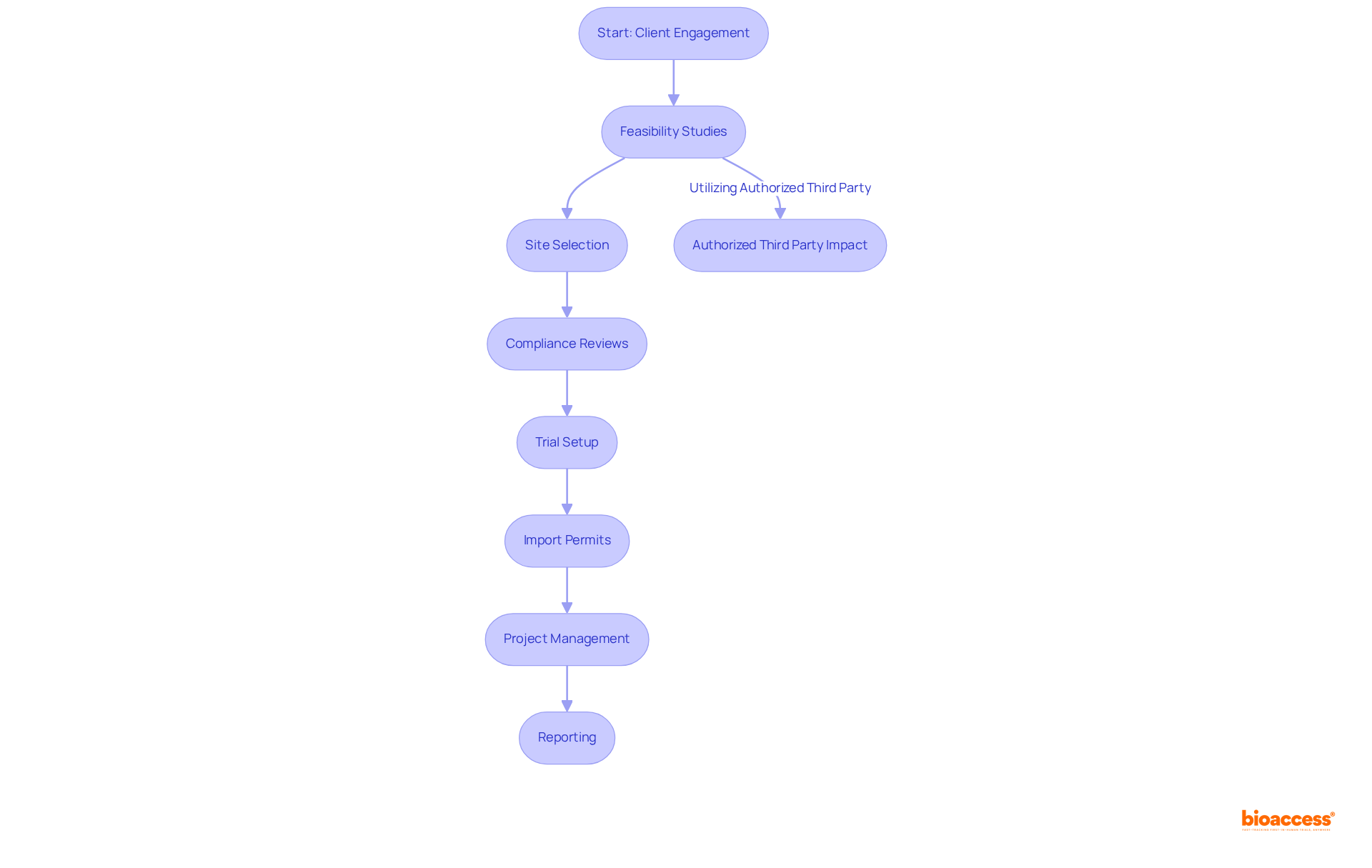
bioaccess® excels in navigating the complexities of COFEPRIS regulations, ensuring that it adheres to the class II device rules COFEPRIS and meets all regulatory requirements. With extensive knowledge of Latin America’s compliance environment, bioaccess® significantly accelerates the approval process, enabling companies to bring their innovations to market more swiftly. Their customized approach includes comprehensive support throughout the registration journey, such as:
This allows clients to focus on their core business while bioaccess® manages the regulatory intricacies. Recent updates indicate that utilizing an Authorized Third Party can reduce the standard review time from 3 to 8 months to just 1 to 3 months, further enhancing entry speed. Regulatory experts emphasize that understanding and navigating these compliance pathways is crucial for successful market entry in Latin America. Additionally, COFEPRIS fees range from $500 to $1,100 USD, providing further context for the financial aspects of the registration process, making bioaccess® an essential ally for Medtech innovators aiming to thrive in this competitive landscape.
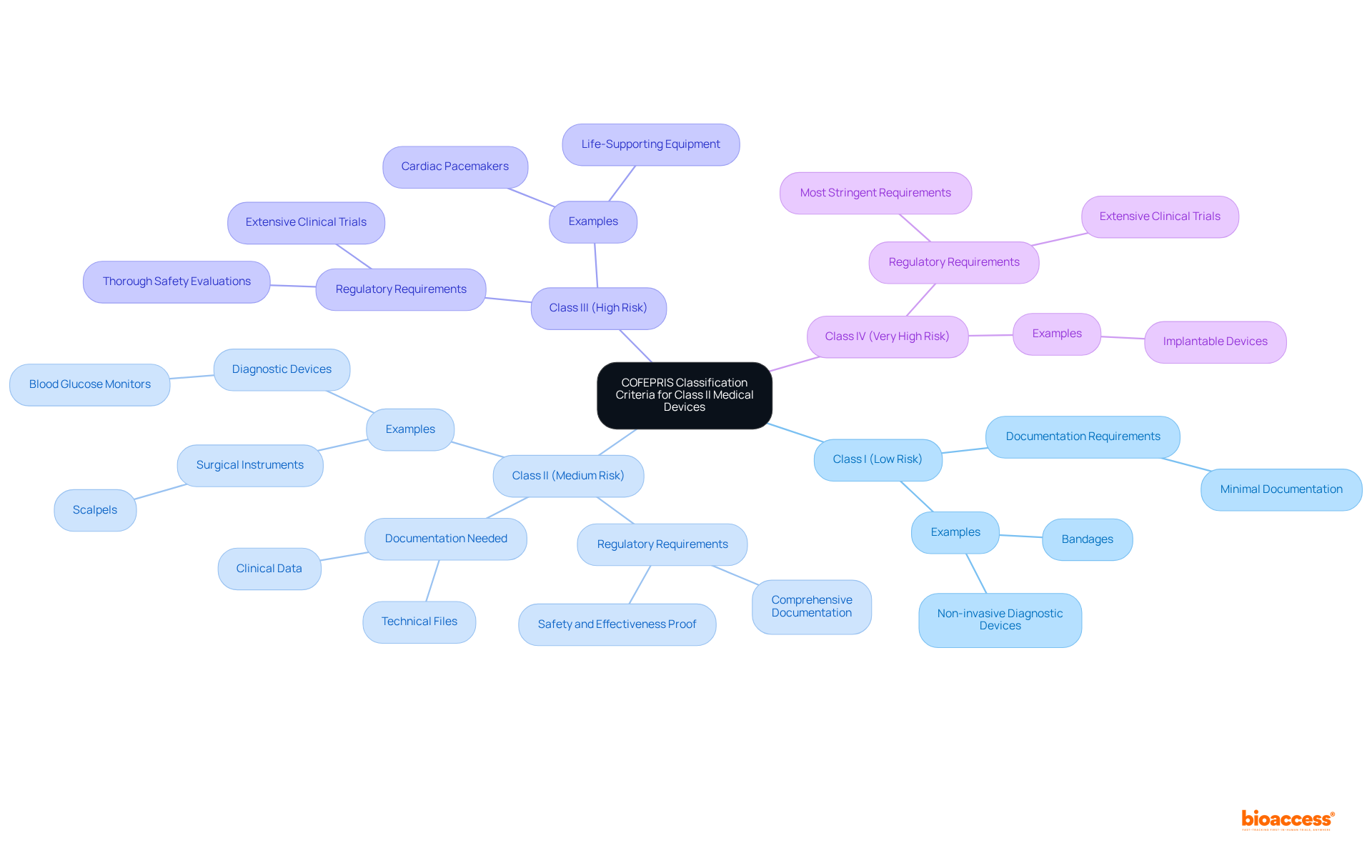
COFEPRIS categorizes medical instruments into four risk-based classifications:
The class II device rules COFEPRIS necessitate a more stringent regulatory process for products encompassing surgical instruments and diagnostic equipment due to their moderate risk profile. Over the past year, there has been a notable increase in the registration of products under the class II device rules COFEPRIS, signaling a growing demand for these items within the Mexican market.
Producers are required to submit comprehensive documentation that demonstrates both the safety and effectiveness of their products, alongside proof of compliance with applicable standards. This documentation includes detailed technical files and clinical data, which are vital for the classification process. As highlighted by industry experts, accurate classification of equipment is crucial for successfully navigating the regulatory landscape. Katherine Ruiz, a compliance specialist, underscores that a thorough understanding of COFEPRIS's classification framework is essential for achieving successful market entry.
Examples of medical equipment that fall under the class II device rules COFEPRIS include surgical instruments like scalpels and diagnostic devices such as blood glucose monitors. The classification process for these instruments involves an exhaustive review of their intended use, safety data, and manufacturing practices. Recent updates to COFEPRIS regulations in 2025 have further refined the classification criteria, ensuring that manufacturers are well-informed about the compliance requirements. This clarity is imperative for expediting the registration process and facilitating prompt market access.
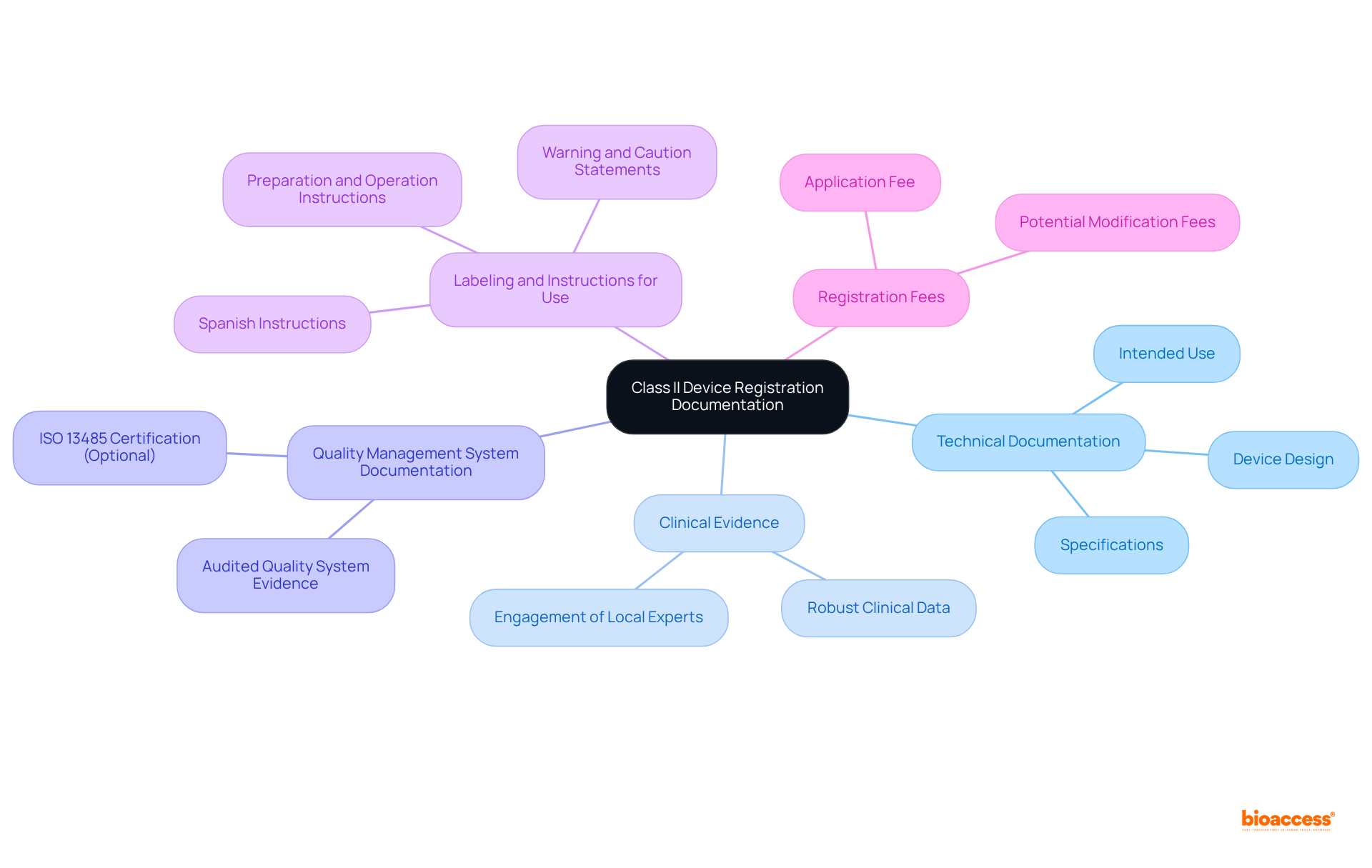
To successfully register a Class II medical device, manufacturers must adhere to the Class II device rules COFEPRIS and compile a detailed registration dossier that includes essential components. The first is Technical Documentation, which outlines the device's design, intended use, and relevant specifications. Next, Clinical Evidence is crucial; manufacturers must provide robust clinical data demonstrating the device's safety and effectiveness, which is essential for official approval. Engaging local experts can significantly enhance the quality of this evidence, ensuring it meets COFEPRIS's expectations. Additionally, Quality Management System (QMS) Documentation is required. Although ISO 13485 certification is not obligatory, evidence of an audited quality system is necessary to ensure compliance with standards, particularly in Latin America, where local nuances may affect regulatory expectations. Furthermore, Labeling and Instructions for Use must be in Spanish, providing clear instructions for safe and proper device use, including preparation, operation, disposal, and necessary warning and caution statements. Cultural sensitivity in communication is vital to ensure understanding and compliance. Finally, Registration Fees must be paid to initiate the registration process.
Thorough documentation is vital, as incomplete or inaccurate submissions are common reasons for rejection under the Class II device rules COFEPRIS. Engaging regulatory consultants, such as those from bioaccess®, can significantly enhance the quality of the dossier, ensuring adherence to local regulations and improving the chances of a successful application. This comprehensive documentation method, combined with regional knowledge, can significantly reduce the time to approval, enabling quicker access to the market for innovative medical products.
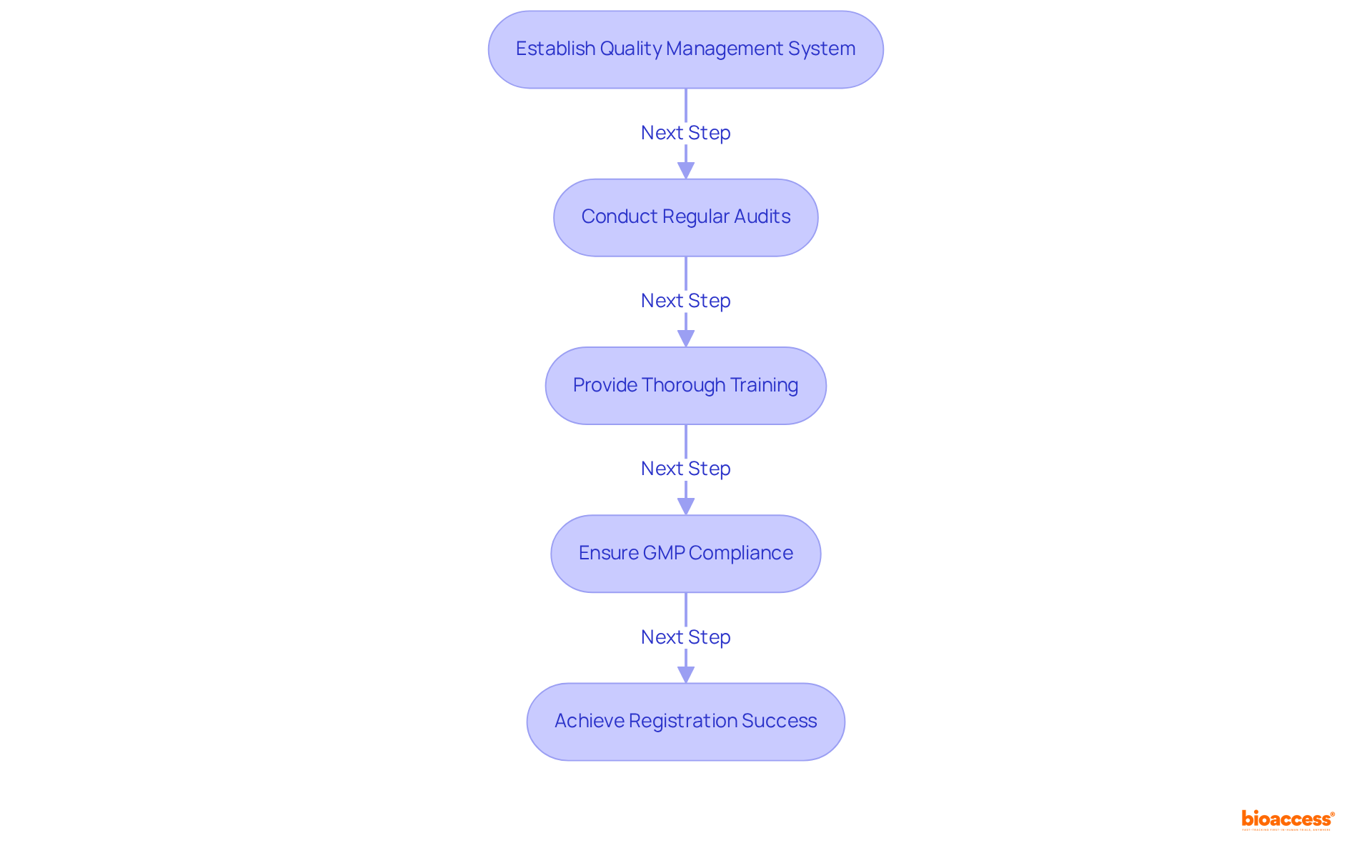
Good Manufacturing Practices (GMP) are crucial for ensuring that medical devices comply with the class II device rules COFEPRIS to consistently meet stringent quality standards. COFEPRIS mandates that manufacturers demonstrate adherence to the class II device rules COFEPRIS, which encompass the establishment of a comprehensive quality management system, regular audits, and thorough training for personnel. This adherence is not merely a regulatory formality; it significantly influences registration outcomes.
Non-compliance can lead to substantial delays or outright rejection of applications, emphasizing the necessity for manufacturers to prioritize GMP throughout the production process. In practice, the impact of GMP compliance on registration success rates for Class II products is profound, as it directly correlates with the efficiency of the class II device rules COFEPRIS registration process. By upholding rigorous GMP standards, manufacturers can enhance their likelihood of successful registration and expedite their entry into the industry.
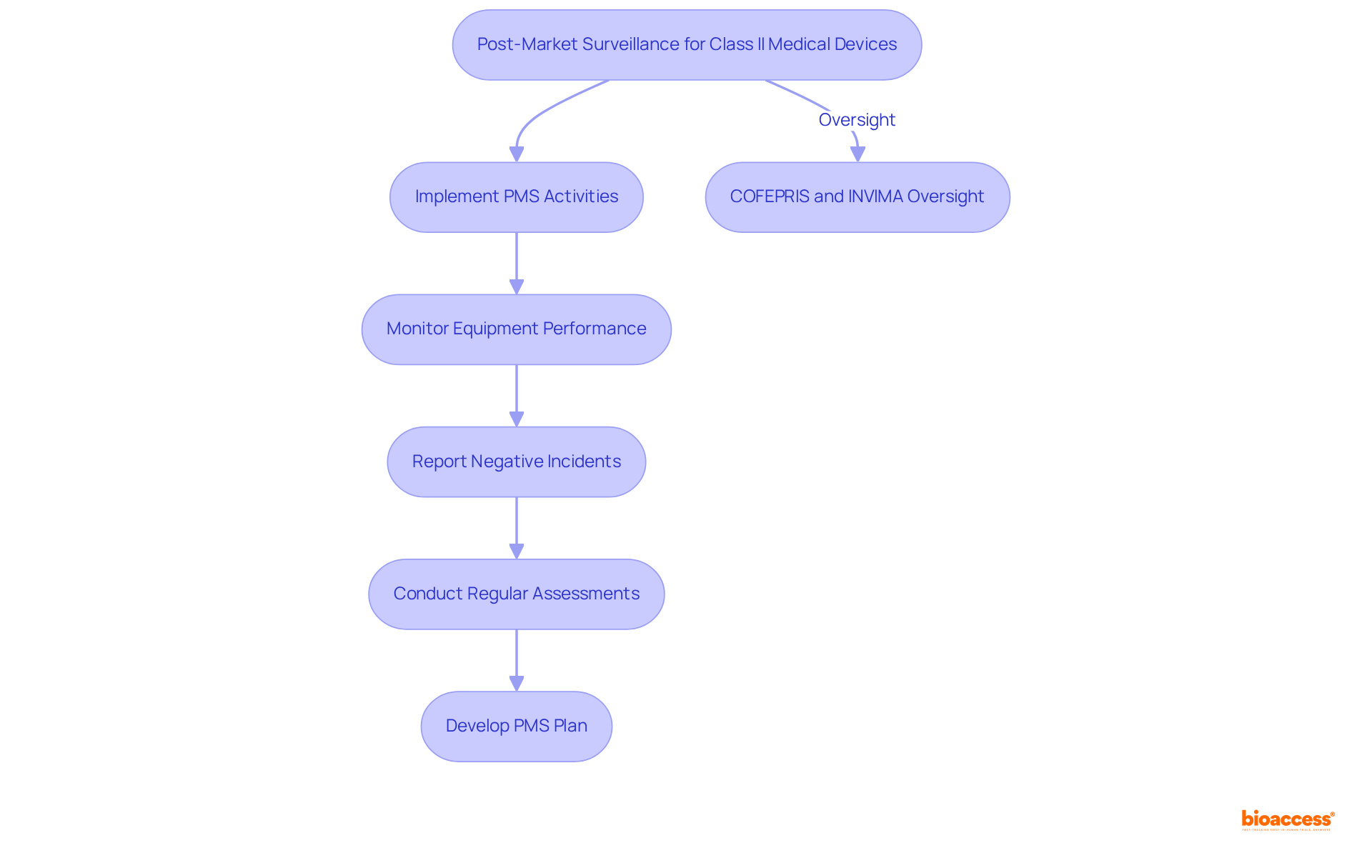
Once a Class II medical product is registered and available in the market, manufacturers must implement post-market surveillance (PMS) activities. This critical process involves overseeing the equipment's performance, reporting negative incidents, and conducting regular assessments to ensure continued safety and effectiveness.
COFEPRIS requires that manufacturers adhere to the Class II device rules by developing a PMS plan that outlines how they will gather and evaluate data related to their products. In a similar vein, INVIMA (Instituto Nacional de Vigilancia de Medicamentos y Alimentos) in Colombia supervises the framework for medical instruments, ensuring adherence to health standards and best practices.
The Directorate for Medical Instruments at INVIMA oversees and regulates these items, underscoring the significance of PMS in ensuring safety and effectiveness. Non-compliance with PMS obligations can result in enforcement actions, including fines or product recalls.
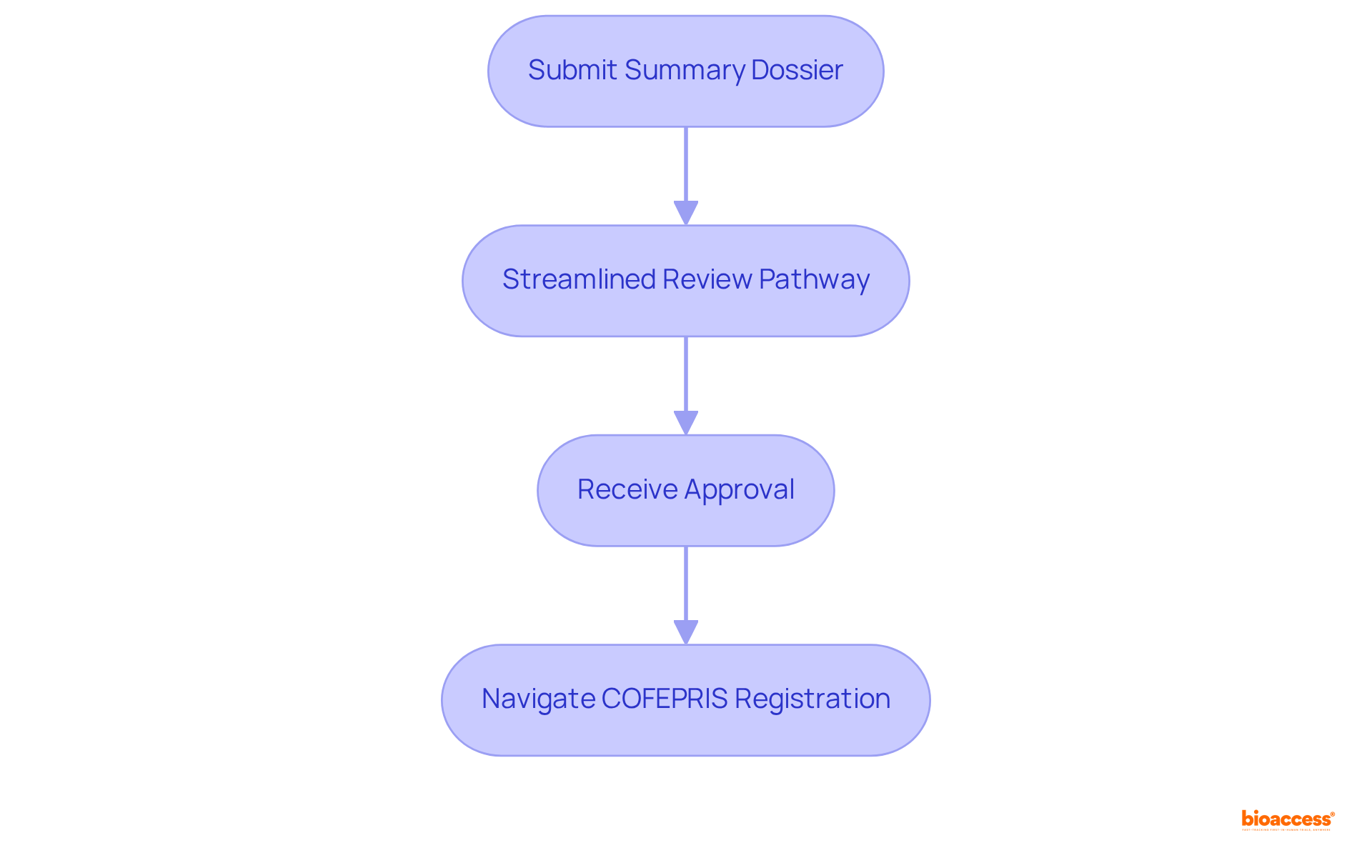
The equivalency review process enables manufacturers to expedite the registration of Class II products already authorized by recognized regulatory bodies, such as the FDA or Health Canada. By submitting a Summary Dossier with less detailed technical information, companies can leverage a streamlined review pathway. This approach is particularly beneficial for those aiming to enter the Mexican market swiftly, as it can save an average of several months in approval time compared to conventional registration methods.
Notably, in 2022, 67 percent of FDA 510(k) submissions resulted in requests for additional information, underscoring the challenges manufacturers face and the critical role of the equivalency review process in alleviating these issues. Industry leaders assert that streamlining registration through equivalency reviews not only accelerates the approval process but also reduces costs associated with lengthy submissions.
By effectively navigating COFEPRIS registration in accordance with the class II device rules COFEPRIS and using equivalency reviews, manufacturers can secure a more efficient route to commercialization, capitalizing on the growing demand for medical devices in Mexico. Producers are encouraged to consider employing equivalency evaluations as a strategic approach for quicker entry into the industry.
Furthermore, partnering with bioaccess can equip manufacturers with the necessary expertise in clinical trial management services, ensuring a smoother navigation through the equivalency review process and enhancing their competitive edge in the market.
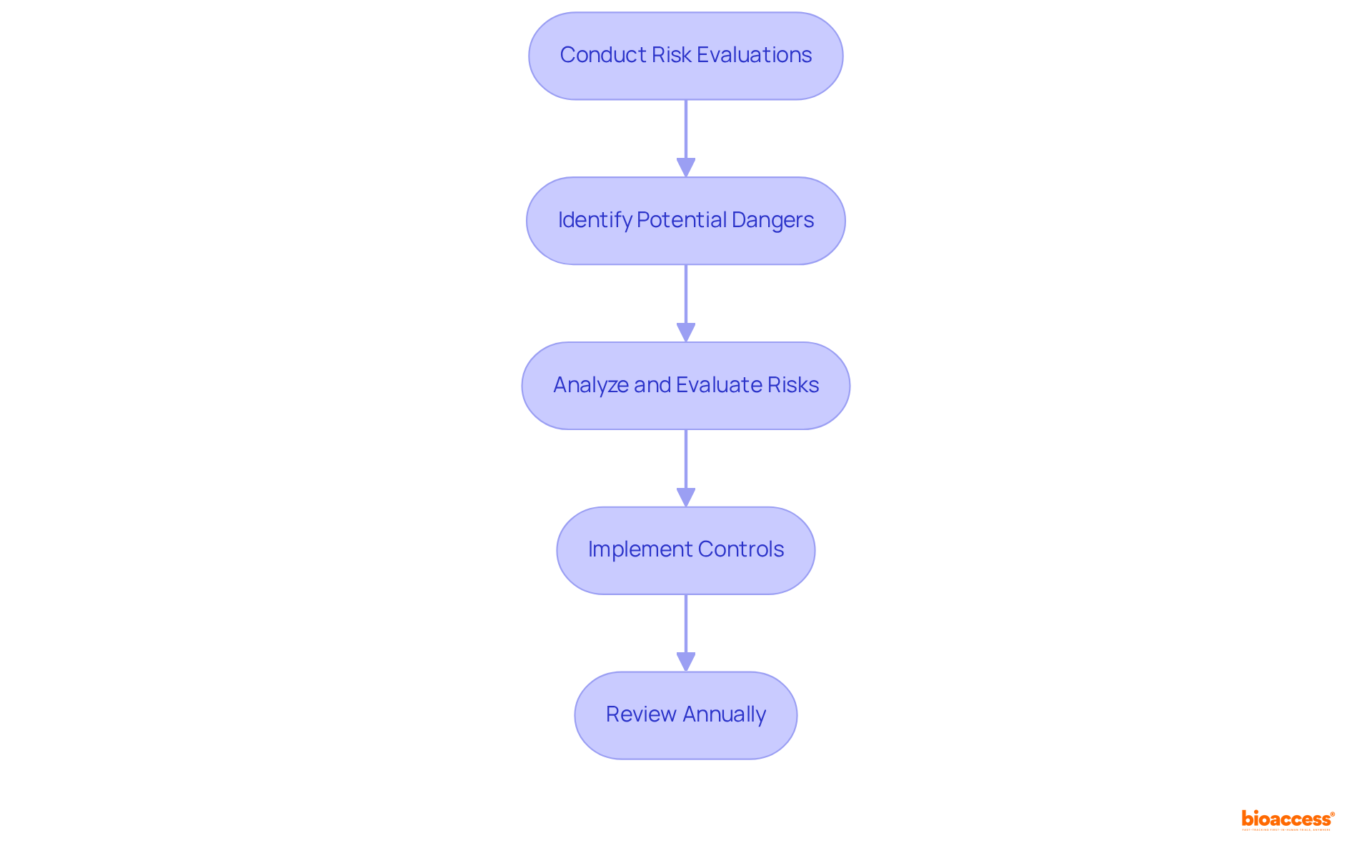
Implementing robust risk management strategies is crucial for the successful development and registration of medical products in accordance with the class II device rules COFEPRIS. Producers must conduct thorough risk evaluations to recognize potential dangers linked to their products, ensuring adherence to ISO 14971 standards, which outline the requirements for risk management throughout the product lifecycle. This standard is acknowledged globally, with major authorities, including INVIMA in Colombia, recognizing its significance in medical equipment risk management. INVIMA, recognized as a Level 4 health authority by PAHO/WHO, supervises the safety and efficacy of medical equipment through its Directorate for Medical Equipment, which oversees compliance, proposes technical standards, and assesses pre- and post-market programs.
Taking initiative in managing risks not only enhances product safety but also significantly increases the chances of successful registration under the class II device rules COFEPRIS in Mexico, which relies on comparable compliance frameworks. Notably, over 54.5% of medical equipment recalls are associated with design problems that could have been prevented with effective risk evaluation procedures, underscoring its essential role in the oversight process. As Jerome F. Lederer states, "Risk management is a more realistic term than safety. It implies that hazards are ever-present, that they must be identified, analyzed, evaluated and controlled or rationally accepted." By implementing effective risk management practices, manufacturers can navigate the complexities of product development while safeguarding patient safety and meeting regulatory expectations. Furthermore, ISO 14971:2019 particularly emphasizes Software as Medical Device (SaMD) applications, and risk management activities must be revised annually at a minimum to uphold regulations.
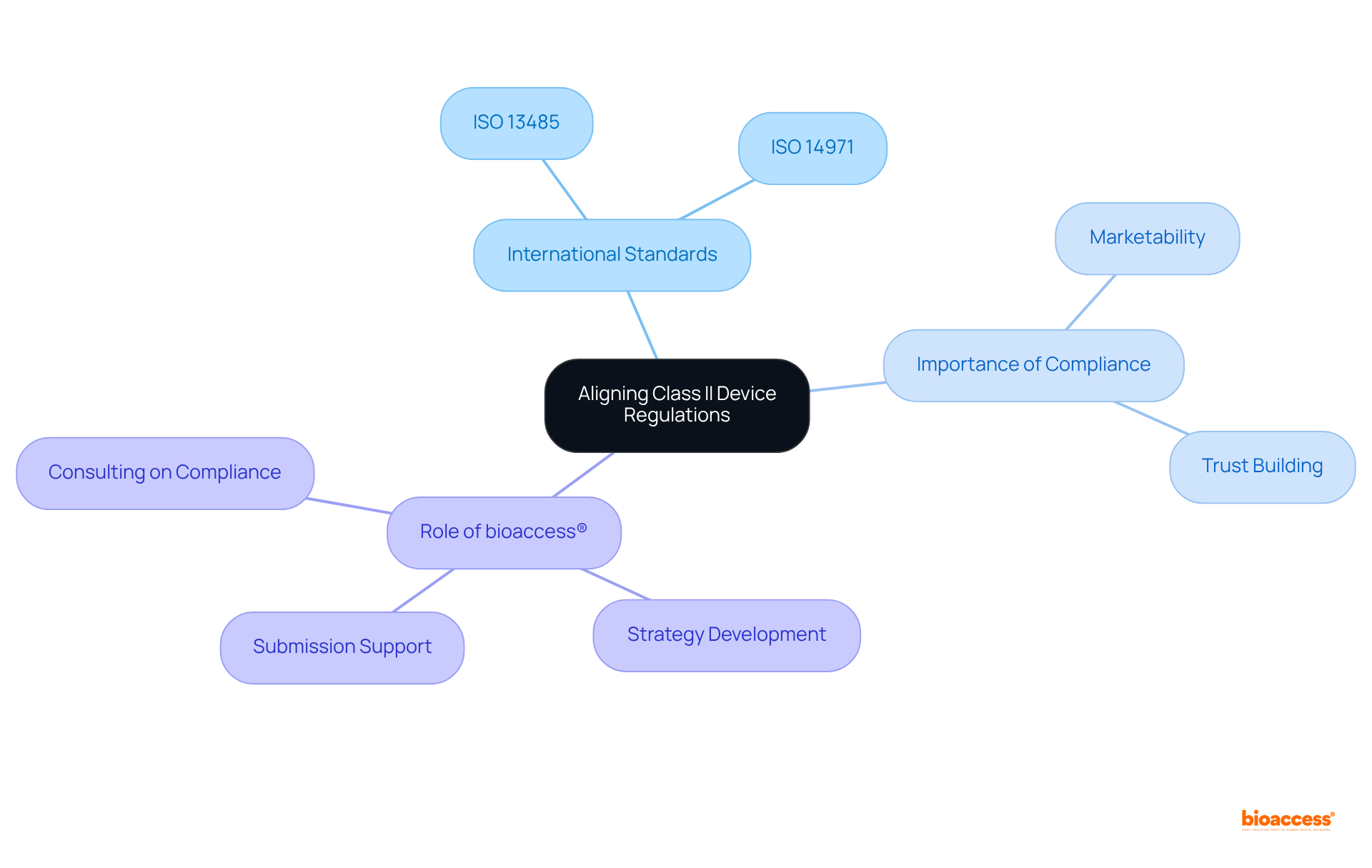
Aligning the class II device rules COFEPRIS with international standards, such as ISO 13485 and ISO 14971, is essential for manufacturers seeking adherence and improved product quality. Compliance with the class II device rules COFEPRIS not only demonstrates a commitment to safety and efficacy but also facilitates smoother interactions with regulatory bodies.
In Mexico, approximately 70% of Class II products conform to international standards, which underscores the importance of the class II device rules COFEPRIS for ISO 13485 compliance. This alignment enhances the marketability of devices in various regions, as numerous countries recognize these certifications as indicators of quality and adherence.
Regulatory specialists assert that following ISO 13485 and ISO 14971 streamlines the approval process and builds trust among stakeholders, ultimately leading to better patient outcomes and increased access to opportunities.
As a leading contract research organization (CRO) in Latin America, bioaccess® provides tailored solutions for Medtech startups, including:
Leveraging its expertise in navigating regulations, bioaccess® ensures successful market entry for its clients. Furthermore, bioaccess®'s recent achievement of ACRP certification represents a pivotal milestone in elevating clinical research standards in the region, reinforcing its dedication to excellence in clinical trials.
For Medtech startups looking to navigate the complexities of regulatory compliance, partnering with bioaccess® can be a strategic move to enhance market access and ensure product success.
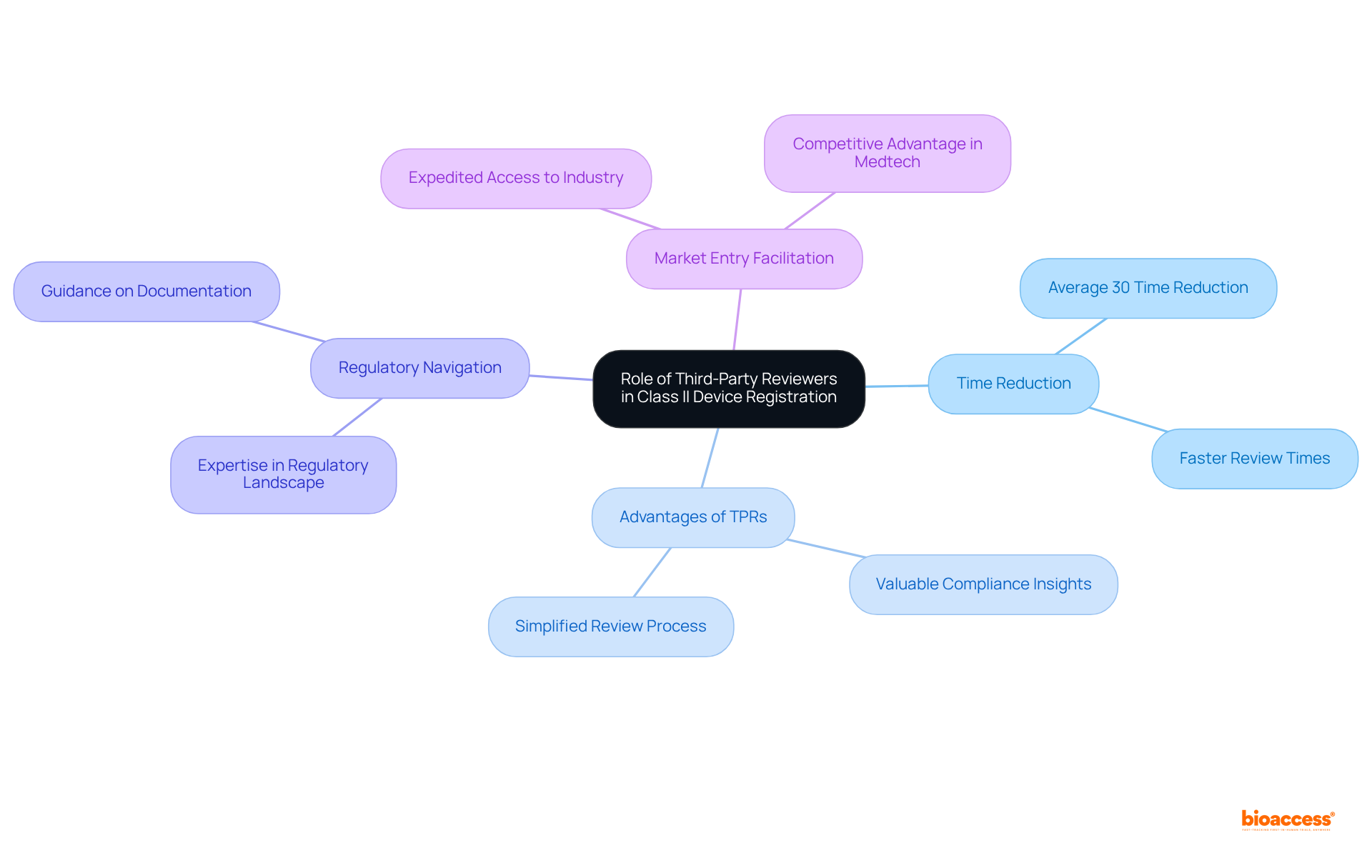
Third-party reviewers (TPRs) play a critical role in the registration process under the class II device rules COFEPRIS, acting as authorized entities that conduct essential pre-reviews of registration dossiers. This process significantly accelerates the overall approval timeline, with TPRs often achieving average time reductions of up to 30% compared to traditional reviews. By leveraging the expertise of TPRs, manufacturers can navigate the complexities of the Mexican regulatory landscape more efficiently, ensuring compliance and enhancing the likelihood of successful approvals.
Successful partnerships with TPRs yield tangible advantages for companies aiming to penetrate the industry. For example, manufacturers utilizing TPRs for their Class II device applications have reported median review times that are markedly shorter than those of in-house submissions. Engaging TPRs not only simplifies the review process but also equips manufacturers with valuable insights into compliance needs, ultimately facilitating a smoother entry into the market.
Industry leaders recognize the substantial benefits of involving TPRs in the oversight process. Their ability to provide pre-approval evaluations and guidance on documentation can lead to expedited access to the industry, which is vital in the competitive Medtech environment. As the regulatory landscape continues to evolve, the role of TPRs in accelerating approvals under the class II device rules COFEPRIS remains an essential strategy for manufacturers seeking to capitalize on opportunities within Mexico's burgeoning healthcare market.
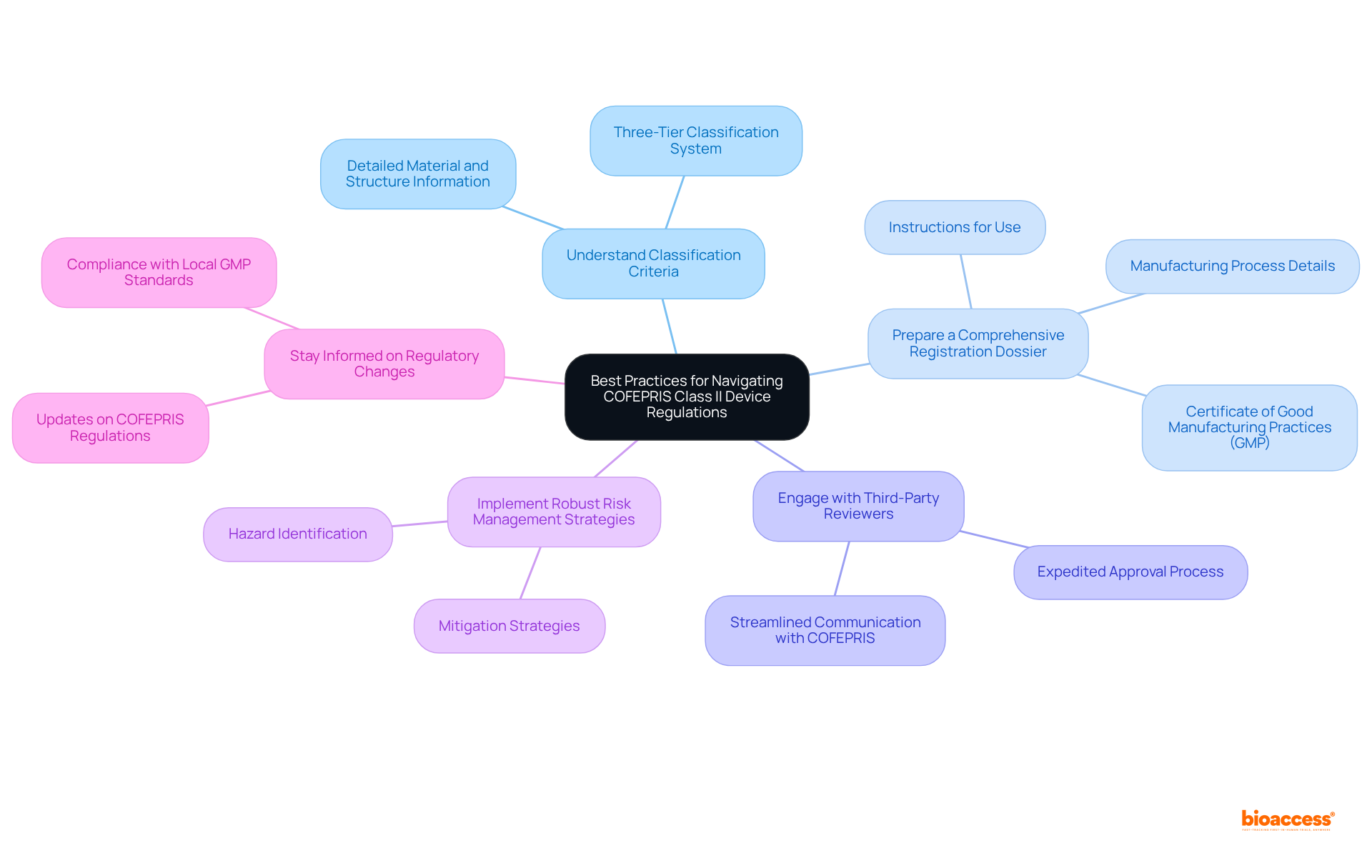
To effectively navigate the class II device rules COFEPRIS, manufacturers must embrace best practices that enhance compliance and market readiness.
Understand Classification Criteria: Accurately categorizing the device is paramount, as Mexico employs a three-tier classification system based on risk. Comprehensive details regarding materials and device structure are required under the class II device rules COFEPRIS, making precise classification essential for adherence.
Prepare a Comprehensive Registration Dossier: A meticulously organized dossier that fulfills all documentation requirements is vital. This includes providing instructions for use, detailed manufacturing processes, and a Certificate of Good Manufacturing Practices (GMP).
Engage with Third-Party Reviewers: Leveraging independent, accredited Third Party Reviewers (TPRs) can significantly expedite the approval process. While this may incur additional costs, TPRs streamline communication with COFEPRIS and facilitate quicker approvals.
Implement Robust Risk Management Strategies: Identifying and mitigating potential hazards through effective risk management is crucial. This proactive approach not only enhances safety but also aligns with regulatory expectations.
Stay Informed on Regulatory Changes: Keeping abreast of updates in COFEPRIS regulations and maintaining compliance with local GMP standards is essential for sustained success in the industry.
By adhering to these best practices and the class II device rules COFEPRIS, manufacturers can markedly improve their chances of successful registration and timely market entry in Mexico, where the medical device market is poised for substantial growth.
Understanding and adhering to the class II device rules set forth by COFEPRIS is essential for manufacturers aiming to navigate the complex landscape of medical device registration in Mexico. This regulatory framework not only ensures that products meet safety and efficacy standards but also positions companies for successful market entry amidst the growing demand for medical innovations.
The article highlights several critical aspects of COFEPRIS regulations, including:
Engaging with Authorized Third Parties can significantly expedite the approval process, while implementing robust risk management strategies is crucial for compliance and product safety. Additionally, aligning with international standards enhances credibility and marketability, making it imperative for manufacturers to stay informed about evolving regulations.
As the medical device market in Mexico continues to expand, manufacturers are encouraged to adopt best practices that not only facilitate compliance with COFEPRIS but also enhance their competitive edge. By leveraging expert support, such as that offered by bioaccess®, and prioritizing thorough documentation and risk management, companies can streamline their registration processes and successfully bring their innovations to market. Embracing these strategies is vital for those aiming to thrive in this dynamic industry.
What is bioaccess® and how does it assist with COFEPRIS regulations?
bioaccess® specializes in navigating COFEPRIS regulations for Class II medical devices, ensuring compliance with all regulatory requirements and accelerating the approval process for companies looking to bring their innovations to market.
What services does bioaccess® provide during the registration journey?
bioaccess® offers a customized approach that includes feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting, allowing clients to focus on their core business while managing regulatory intricacies.
How can using an Authorized Third Party impact the review time for COFEPRIS?
Utilizing an Authorized Third Party can reduce the standard review time from 3 to 8 months down to just 1 to 3 months, enhancing the speed of market entry.
What are the COFEPRIS classification criteria for medical devices?
COFEPRIS categorizes medical devices into four classifications based on risk: Class I (low risk), Class II (medium risk), Class III (high risk), and Class IV (very high risk). Class II devices, which include surgical instruments and diagnostic equipment, undergo a more stringent regulatory process.
What documentation is required for Class II device registration with COFEPRIS?
Required documentation includes Technical Documentation (device design and specifications), Clinical Evidence (data on safety and effectiveness), Quality Management System (QMS) Documentation, Labeling and Instructions for Use in Spanish, and payment of Registration Fees.
Why is accurate classification of medical devices important?
Accurate classification is crucial for successfully navigating the regulatory landscape, as it determines the regulatory requirements and processes that manufacturers must follow for their products.
What are some examples of Class II medical devices?
Examples of Class II devices include surgical instruments like scalpels and diagnostic devices such as blood glucose monitors.
What role does cultural sensitivity play in the registration process?
Cultural sensitivity in communication is vital to ensure that labeling and instructions for use are clear and understood, which is essential for compliance and safe device usage.
How can engaging regulatory consultants like bioaccess® benefit manufacturers?
Engaging regulatory consultants can enhance the quality of the registration dossier, ensuring adherence to local regulations and improving the chances of a successful application, thereby reducing the time to approval.