


Achieving CE approval for medical devices is a structured and critical process that encompasses:
This process is essential for ensuring compliance with EU regulations, which not only facilitates market entry but also significantly enhances consumer trust in the safety and efficacy of medical products.
Understanding this framework is vital for stakeholders in the Medtech landscape, as it addresses key challenges in clinical research and underscores the importance of regulatory adherence.
Achieving CE approval for medical devices is a crucial step for manufacturers aiming to enter the European market, as it signifies compliance with essential health and safety standards. This guide offers a comprehensive roadmap to navigate the complexities of the CE marking process, from understanding its significance to engaging with notified bodies for certification. With stringent regulations and potential pitfalls ahead, manufacturers must consider:
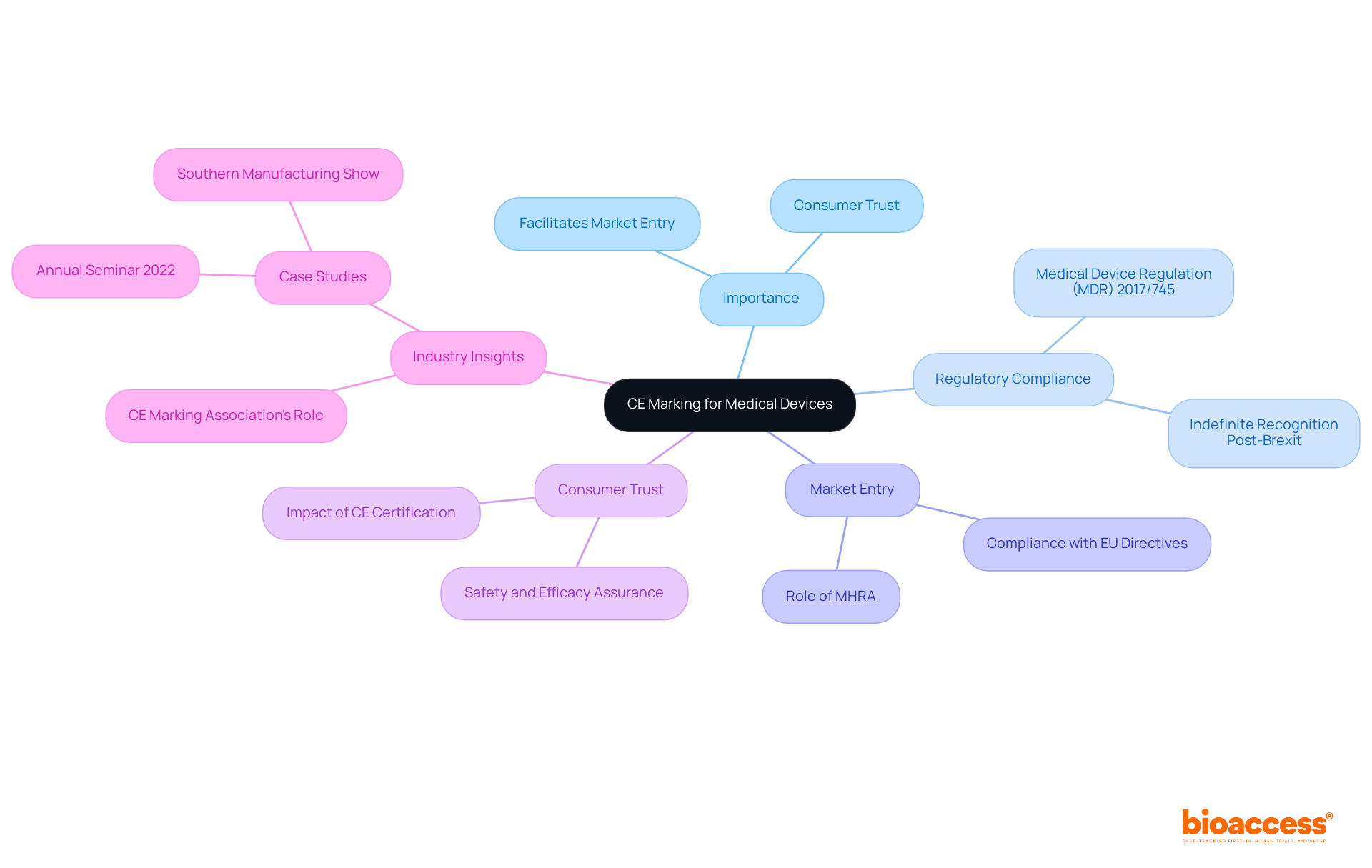
CE approval for medical devices, or Conformité Européenne, serves as a critical acknowledgment that signifies a medical product's conformity with essential health, safety, and environmental protection standards established by the European Union (EU). CE approval for medical devices is mandatory for all medical products marketed within the European Economic Area (EEA). The CE approval for medical devices indicates that the manufacturer has conducted a thorough assessment of the product, ensuring compliance with all relevant EU directives, particularly the Medical Device Regulation (MDR) 2017/745.
The significance of CE certification extends beyond mere compliance; it is a vital facilitator of market entry and a cornerstone in fostering consumer trust regarding the safety and efficacy of medical products. Industry leaders emphasize that without CE approval for medical devices, a medical product cannot be legally marketed in the EU, underscoring its indispensable role in the commercialization process. For instance, the CE Marking Association has pointed out that the indefinite recognition of the CE mark post-Brexit offers businesses continuity and flexibility, enabling them to concentrate on innovation and growth. Notably, the CE mark will be recognized indefinitely in 18 areas, reinforcing its wide-ranging applicability and importance.
Moreover, recent case studies illustrate the benefits of CE certification for manufacturers. The updated 'Blue Guide' published by the European Commission acts as a vital resource for businesses, ensuring adherence to the latest regulations and facilitating smoother market entry. Additionally, the MHRA's commitment to recognizing CE-marked products indefinitely enhances the regulatory landscape, allowing for quicker access to innovative medical technologies. The CE Marking Association's participation in the Southern Manufacturing Show provided valuable insights into the CE certification process and recent legislative changes, highlighting the industry's proactive approach to compliance.
In conclusion, understanding and achieving CE approval for medical devices is not just a regulatory hurdle; it serves as a strategic advantage that can significantly impact a manufacturer's ability to effectively market their medical products within the competitive EU landscape. As Kevin Hollinrake, the business minister, stated, 'By broadening CE certification use throughout the UK, companies can concentrate their time and resources on generating jobs and enhancing the economy.
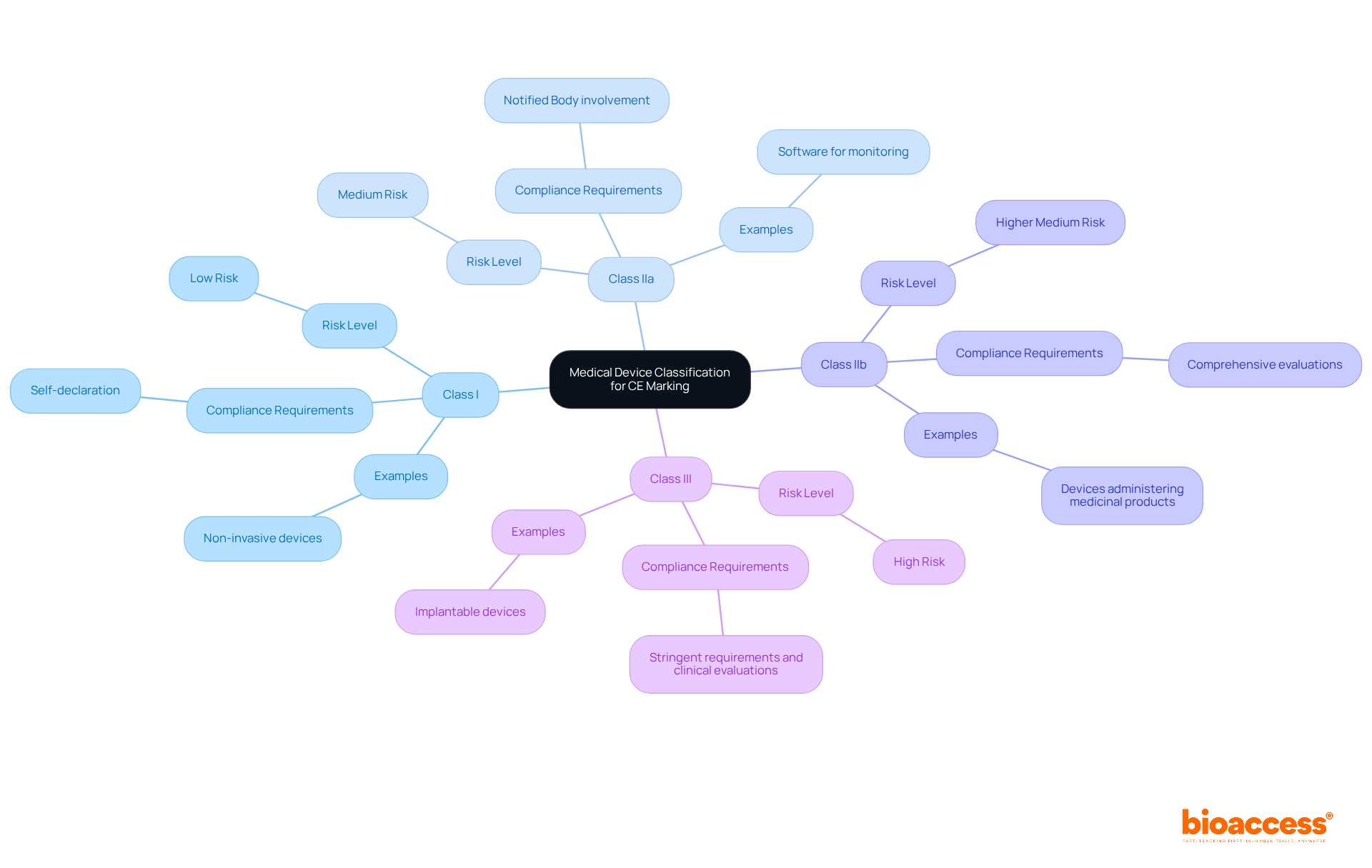
The initial step in the CE marking process is to categorize your medical product according to the EU classification rules. Medical instruments are classified into four categories based on their risk levels:
This classification is influenced by several factors, including the intended purpose of the item, duration of contact with the body, and its invasiveness. According to the EU Medical Device Regulation (MDR), the classification rules are detailed in Annex VIII, which outlines 22 specific rules for determining the suitable category for each apparatus.
For example, Class III products, regarded as high risk, face the most stringent requirements, including comprehensive clinical evaluations. In contrast, Class I items, which pose minimal risk, can often self-declare compliance with common specifications. Notably, as of 2023, 33% of all certified medical devices are categorized as Class IIb, indicating a significant number of devices that require a more thorough evaluation.
To ensure accurate classification, it is advisable to consult the European Commission's guidance documents or seek expert advice. Engaging with regulatory experts, such as Ana Criado, Director of Regulatory Affairs and a professor with extensive experience in biomedical engineering and health economics, can aid in navigating the complexities of the classification process. Misclassification can lead to delays in acquiring CE approval for medical devices and following the required conformity assessment procedures.
Furthermore, the implementation of a Unique Device Identifier (UDI) is now mandatory, adding another layer of regulatory compliance that manufacturers must consider. The involvement of Notified Bodies is essential for higher-risk categories to achieve CE approval for medical devices, as they evaluate compliance and ensure that products meet safety and effectiveness standards before entering the market.
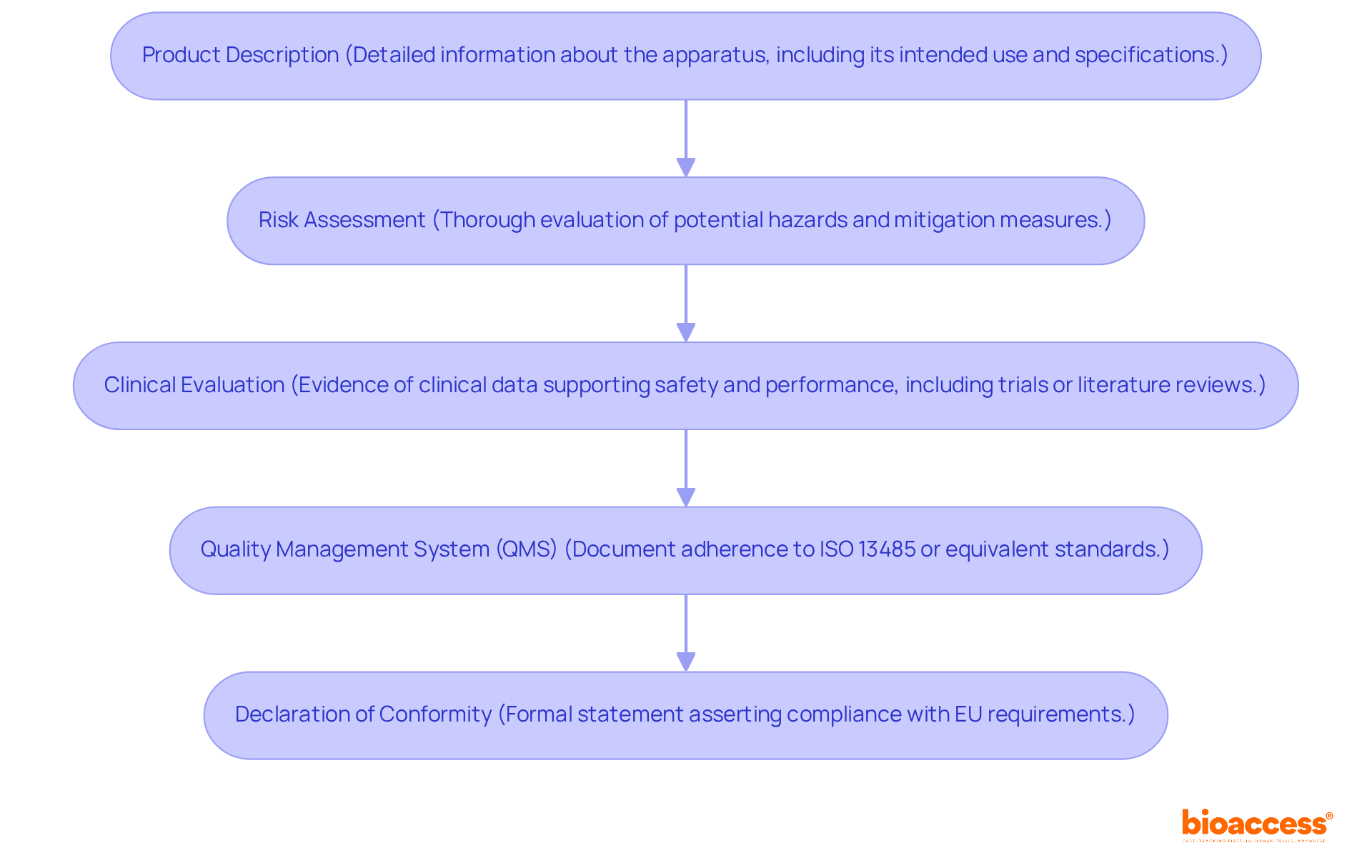
To achieve CE marking, manufacturers must compile a comprehensive technical file that demonstrates compliance with the relevant EU directives. This documentation should include:
It is essential to ensure that all documentation is organized and readily accessible, as over 50% of Technical Documentation submitted to regulatory authorities is deemed incomplete, often resulting in requests for additional information. This underscores the critical importance of meticulous preparation and review to facilitate a smooth assessment process.
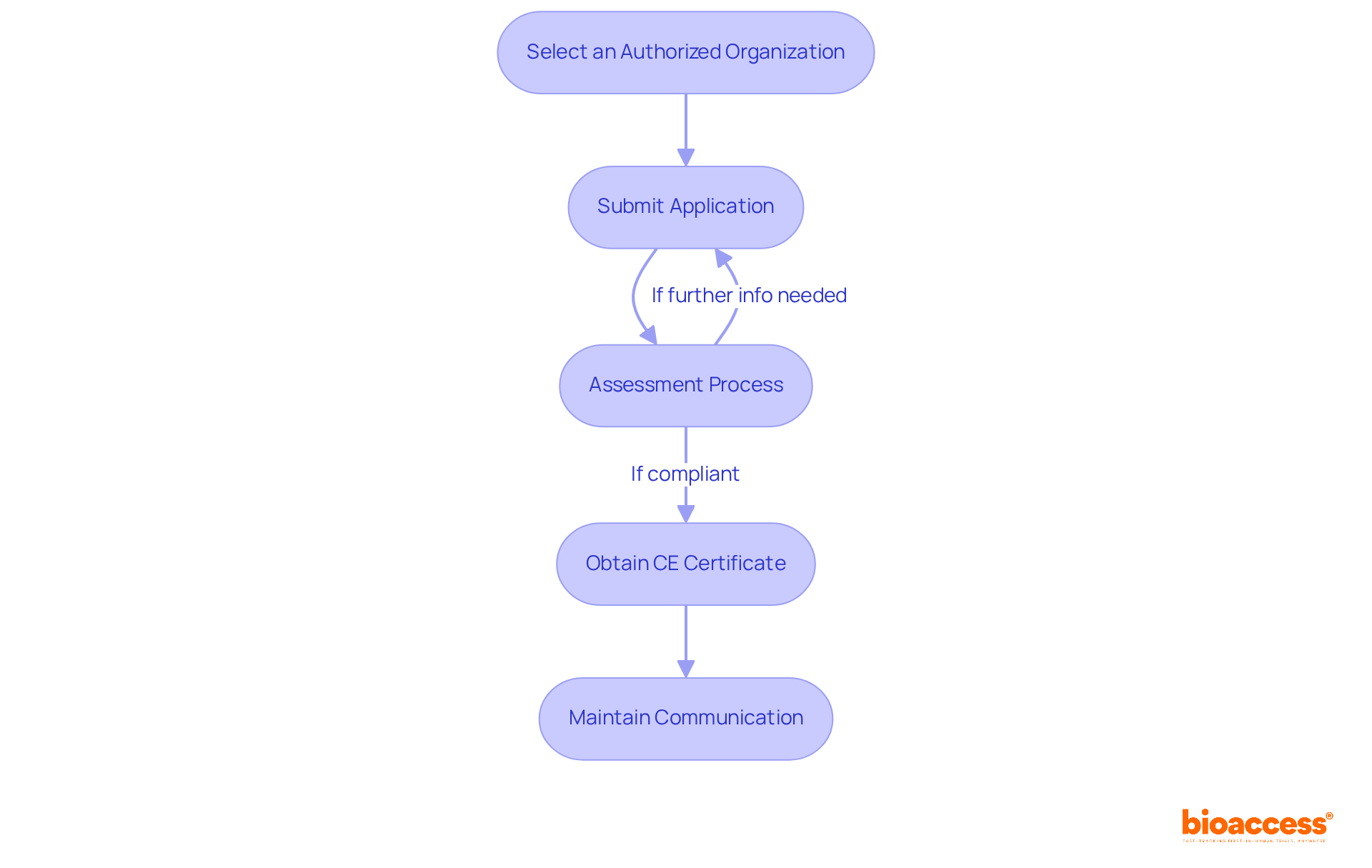
Once the technical documentation is prepared, the subsequent step involves engaging with an authorized body—an organization appointed by EU member states to assess the conformity of medical products prior to obtaining CE approval for medical devices. To initiate this process:
Select an Authorized Organization: Choose an Authorized Organization that corresponds with your device's classification and possesses the requisite expertise. Given that there are currently 50 MDR-certified Organizations, it is crucial to select one that can support your future product strategies, thereby avoiding additional costs associated with changing providers.
Submit Application: Provide the designated body with your technical documentation and any supplementary information they may require. Ensure that your submission is comprehensive to facilitate a smoother review process.
Assessment Process: The Authorized Body will examine your documentation and may conduct an audit of your Quality Management System (QMS). Be prepared for potential inquiries or requests for further information, as designated bodies evaluate conformity through technical, clinical, and quality reviews. Maintaining clear communication lines with the designated body can enhance collaboration and expedite the process.
Obtain CE Certificate: If your equipment meets all criteria, the certifying body will issue a CE certificate, allowing you to affix the CE mark to your product. Obtaining CE approval for medical devices is vital for marketing your device within the EU.
Throughout this procedure, it is prudent to maintain transparent communication with the designated body to swiftly address any issues. Engaging with a single Notified Body can minimize review overlap and streamline the certification process, ultimately leading to a more efficient pathway to market.
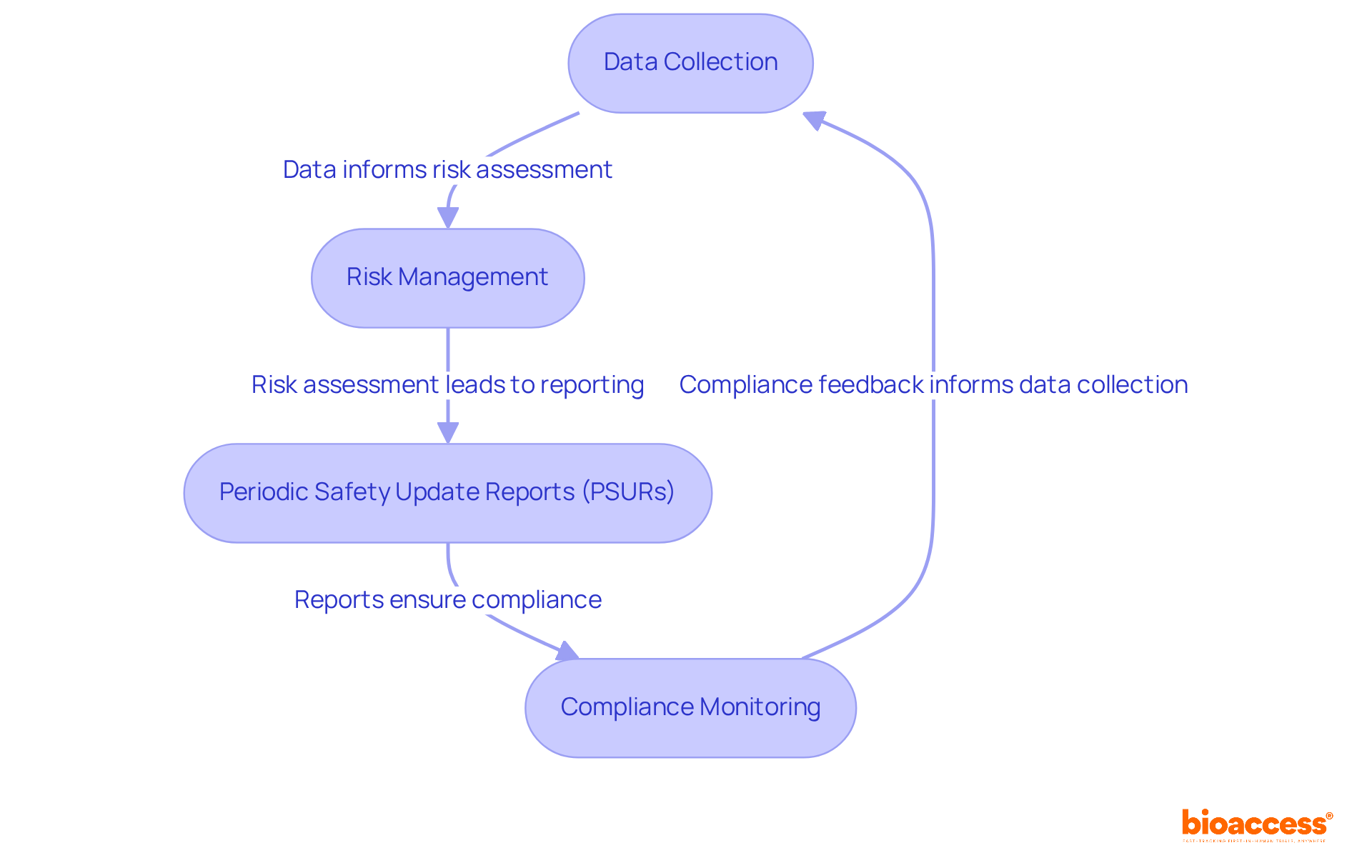
Following the achievement of CE approval for medical devices, manufacturers must establish a thorough post-market surveillance (PMS) system to effectively monitor the safety and performance of their medical products in real-world environments. This entails several key components:
Data Collection: Establish robust systems to gather information on equipment performance, encompassing user feedback, adverse events, and clinical outcomes. Effective data collection is crucial, as it allows manufacturers to identify trends and potential safety issues early. Significantly, an average of 1,000 adverse events are reported each year for CE-marked products, highlighting the necessity of careful monitoring.
Risk Management: Continuously assess risks related to the equipment based on the data gathered. This ongoing assessment is vital for implementing timely corrective actions and ensuring that any emerging risks are addressed proactively. It is also crucial to mention that PMS is necessary for class II and class III medical instruments that pose a potential for serious risk to health.
Periodic Safety Update Reports (PSURs): Manufacturers are required to prepare and submit PSURs to relevant authorities. These reports summarize the safety and performance information collected during the reporting period, offering a clear overview of the product's real-world impact. Furthermore, the clinical evaluation report (CER) must be updated every two to five years for products without significant risk, ensuring ongoing compliance responsibilities.
Compliance Monitoring: Regularly review adherence to regulatory requirements and update documentation as necessary. This includes ensuring that all PMS activities align with the latest regulations and standards, such as ISO 20416:2020, which outlines a systematic process for manufacturers to collect and analyze post-market data to facilitate CE approval for medical devices. This is essential for maintaining market access.
Implementing a proactive PMS system not only guarantees adherence but also greatly improves the overall safety and efficiency of medical products in the market. By fostering a culture of vigilance and continuous improvement, companies can safeguard user health and maintain their reputation in the industry. Furthermore, engaging with users for feedback is crucial, as it can provide valuable insights into device performance and safety.
Achieving CE approval for medical devices is a crucial milestone for manufacturers aspiring to penetrate the European market. This process not only guarantees adherence to vital health and safety standards but also bolsters consumer confidence in the effectiveness of medical products. The CE marking functions as a gateway for manufacturers, affirming their dedication to quality and compliance—an essential factor for successful commercialization within the European Economic Area (EEA).
This guide has meticulously outlined the key elements of the CE approval process, highlighting the significance of:
Furthermore, establishing a robust post-market surveillance system is imperative for ongoing compliance and safety monitoring. Each of these steps contributes to a comprehensive strategy that protects both the manufacturer’s interests and the welfare of end-users.
The importance of CE marking transcends regulatory compliance; it embodies a commitment to quality and safety within the medical device sector. Manufacturers are urged to view this process as an opportunity to amplify their market presence and stimulate innovation. By prioritizing compliance and proactively engaging with regulatory requirements, companies can not only fulfill their legal obligations but also play a pivotal role in advancing healthcare standards, ultimately benefiting patients and the wider community.
What is CE marking and why is it important for medical devices?
CE marking, or Conformité Européenne, signifies that a medical product conforms to essential health, safety, and environmental protection standards established by the European Union (EU). It is mandatory for all medical products marketed within the European Economic Area (EEA) and indicates that the manufacturer has conducted a thorough assessment to ensure compliance with relevant EU directives, particularly the Medical Device Regulation (MDR) 2017/745.
What are the consequences of not having CE approval for medical devices?
Without CE approval, a medical product cannot be legally marketed in the EU, making CE certification an indispensable part of the commercialization process for medical devices.
How does CE certification benefit manufacturers?
CE certification serves as a strategic advantage, facilitating market entry and fostering consumer trust in the safety and efficacy of medical products. It also allows manufacturers to focus on innovation and growth, especially post-Brexit, where the CE mark is recognized indefinitely in multiple areas.
What are the classifications of medical devices for CE marking?
Medical devices are classified into four categories based on risk levels: Class I (low risk), Class IIa (medium risk), Class IIb (higher medium risk), and Class III (high risk).
What factors influence the classification of a medical device?
The classification is influenced by the intended purpose of the device, the duration of contact with the body, and its invasiveness.
What is the significance of the Unique Device Identifier (UDI)?
The UDI is now mandatory for medical devices, adding another layer of regulatory compliance that manufacturers must consider when seeking CE approval.
What role do Notified Bodies play in the CE approval process?
Notified Bodies evaluate compliance for higher-risk categories of medical devices, ensuring that products meet safety and effectiveness standards before they can enter the market.
Where can manufacturers find guidance for the CE marking process?
Manufacturers can consult the European Commission's guidance documents or seek expert advice to navigate the complexities of the classification and CE marking process.