


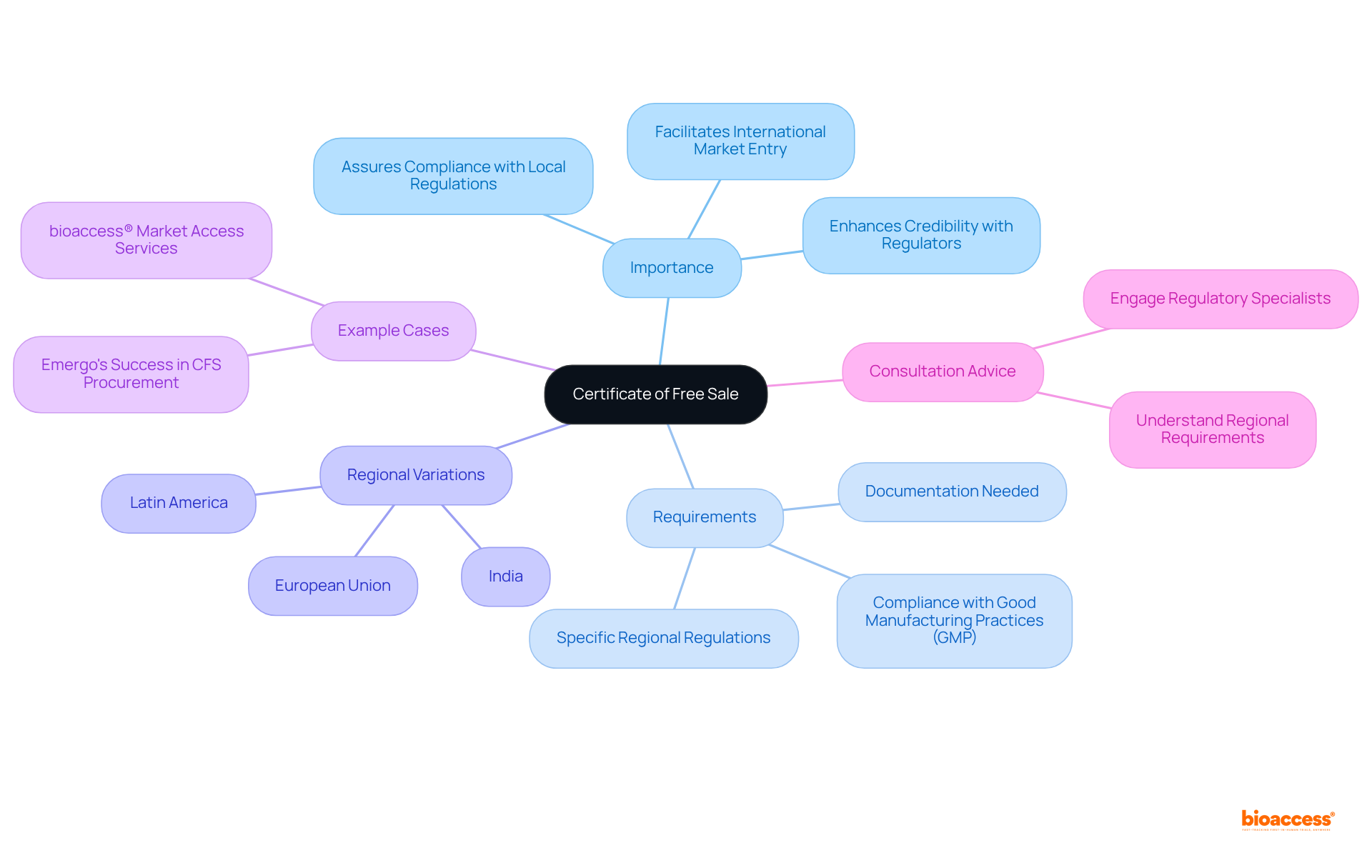
The article emphasizes the essential steps to secure a Certificate of Free Sale (CFS), a critical requirement for companies aiming to export regulated products. Understanding the CFS is paramount, as it lays the groundwork for successful international market entry. The article delineates the necessary documentation for application, the submission process, and troubleshooting common issues. Thorough preparation and strict adherence to local regulations are underscored as vital components for achieving compliance and facilitating export activities.
Navigating the complexities of international trade presents a formidable challenge, particularly when ensuring compliance with diverse regulatory requirements. A pivotal document that can greatly facilitate this process is the Certificate of Free Sale (CFS), which stands as a testament to a product's legitimacy and adherence to local standards. This guide outlines the essential steps for securing a CFS application, equipping businesses with the knowledge necessary to streamline market entry and bolster credibility in foreign markets. However, what occurs when the application process encounters unforeseen challenges, and how can companies adeptly address these obstacles?
A certificate of free sale (CFS) is an official document issued by a government authority, certifying that an item is legally sold in its country of origin and complies with local regulations. This certificate of free sale is often essential for exporting medical devices, pharmaceuticals, and other regulated items, assuring foreign authorities that they meet safety and efficacy standards. Understanding the certificate of free sale is crucial for companies aiming to penetrate international markets, as it helps facilitate smoother customs clearance and fosters trust with foreign regulators and customers.
The certificate of free sale verifies that your product is authorized for sale in your homeland, which is vital for gaining acceptance in overseas markets. In many countries, particularly in regions such as Latin America and the Balkans, the certificate of free sale often serves as a prerequisite for market entry, where regulatory compliance is paramount. For example, in India, the certificate of free sale is required for medical device registration, underscoring its importance in global trade.
Familiarize yourself with the specific requirements of the regions you are targeting, as these can vary significantly. Regulatory specialists emphasize that the certificate of free sale (CFS) serves as a guarantee from the importing nation to the exporting nation regarding the authenticity of the manufacturer and its products, enhancing the credibility of medical devices in global markets. Rebecca Brust, Marketing & Sales Coordinator, asserts that the certificate of free sale (CFS) is a critical document that certifies a specific medical device as an authorized healthcare product in the country of manufacture by the relevant regulatory body.
Companies that have successfully leveraged the certificate of free sale for international market entry include those skilled at navigating the complexities of the Latin American healthcare sector. By utilizing bioaccess®'s market access services to obtain their certificate of free sale, these companies have expedited their entry into lucrative markets. This strategic approach not only ensures compliance but also positions companies advantageously in competitive landscapes. To achieve a successful submission for the certificate of free sale (CFS), it is prudent to consult with experts who can guide you through the specific requirements and processes involved.
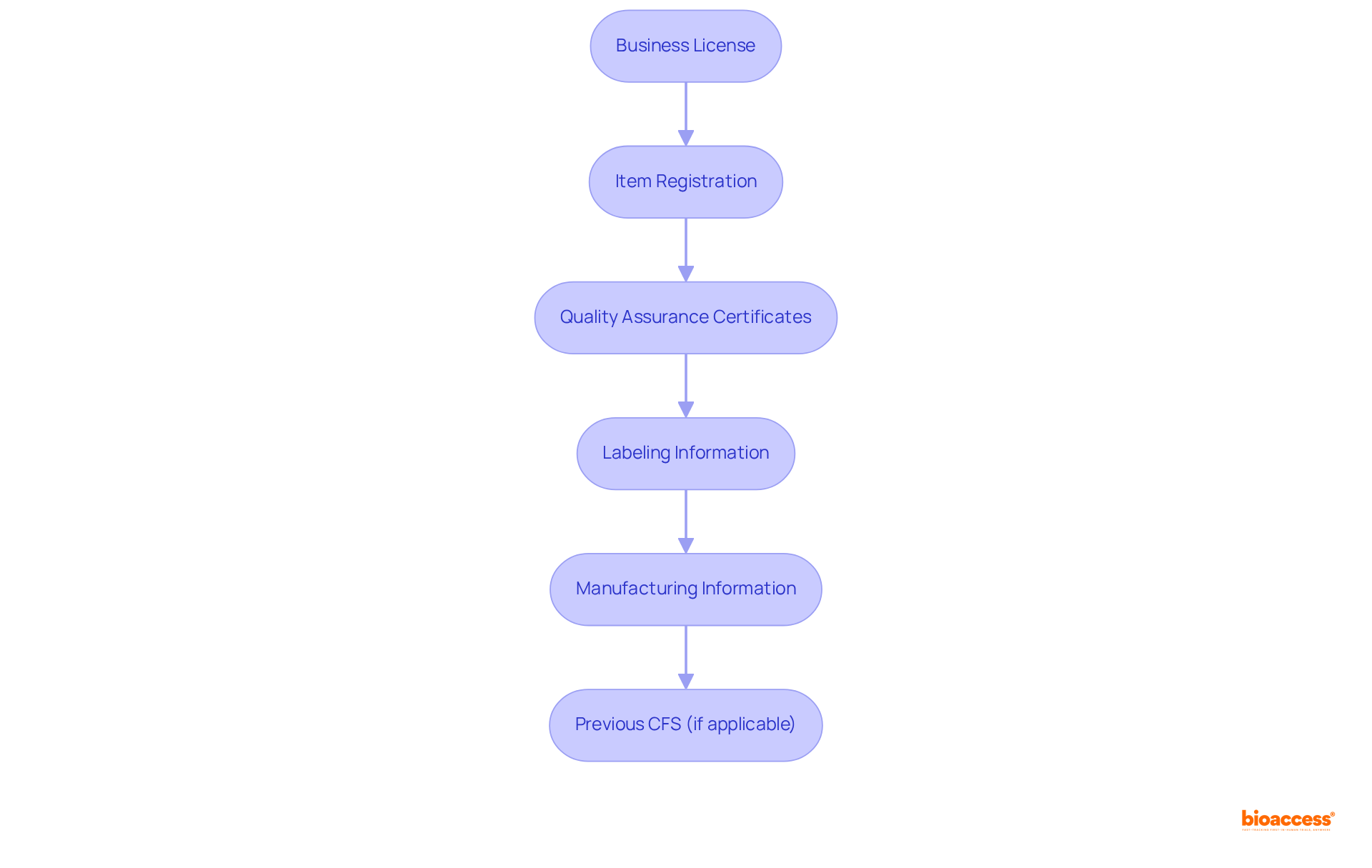
Compiling essential documentation is crucial to successfully apply for a certificate of free sale (CFS). Follow this step-by-step guide to ensure you have everything needed for a smooth application process:
Ensure that all documents are current and accurately represent your goods and business operations. This thorough preparation not only simplifies the submission process but also boosts the credibility of your products in global markets. Remember, acquiring a certificate of free sale usually requires up to 10 business days, and the fee for each certificate is $25.00. As Ben Thompson observes, 'The purpose of a certificate of free sale is to provide assurance to the importing nation about the status and quality of imported goods.' Additionally, be aware that shipments without compliant documentation may be held or rejected by customs authorities.
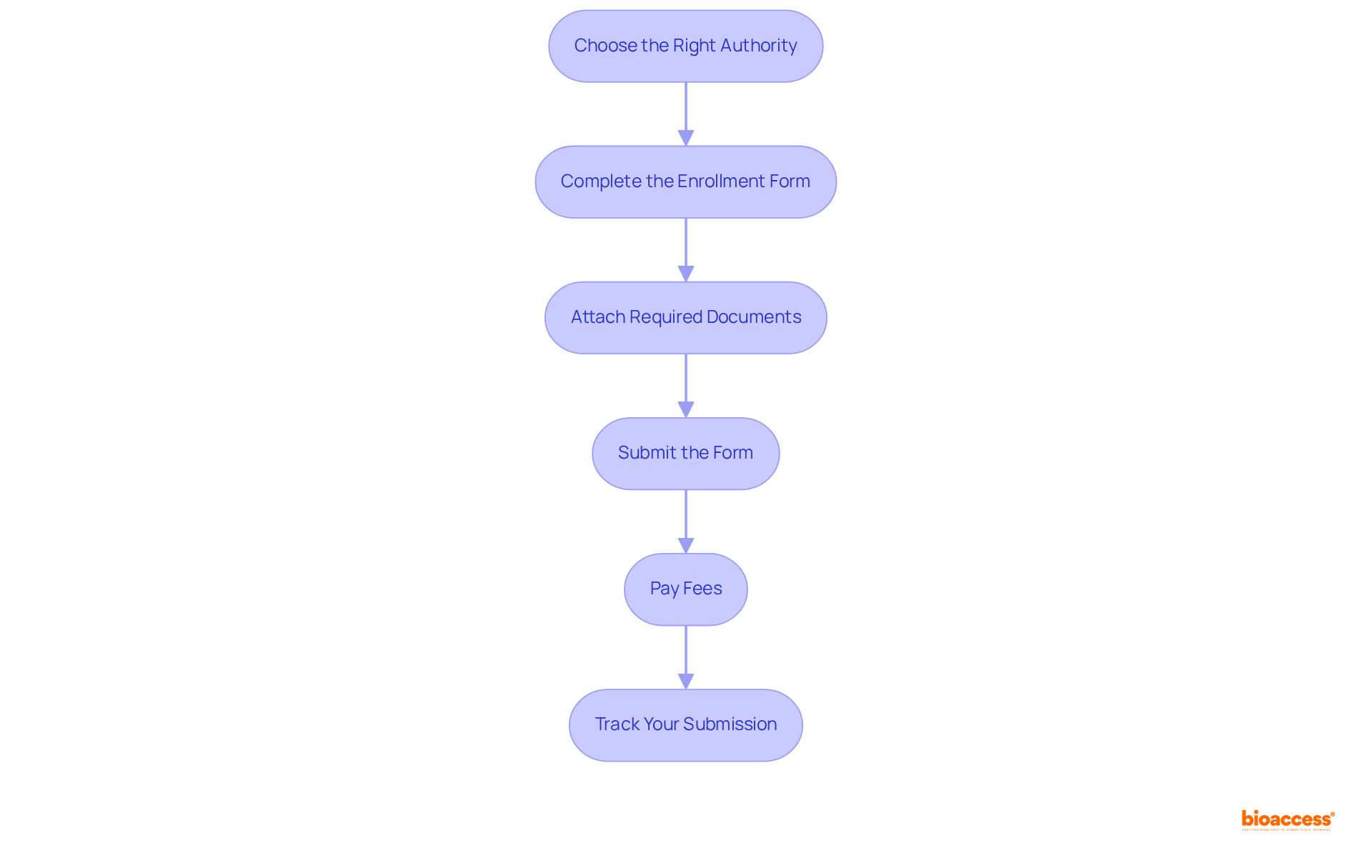
To successfully submit your application for the Certificate of Free Sale (CFS), follow these essential steps:
Choose the Right Authority: Identify the appropriate government agency responsible for issuing the CFS in your region. This may include health departments, regulatory agencies, or trade offices, depending on your country’s requirements.
Complete the Enrollment Form: Accurately fill out the enrollment form, ensuring that all information aligns with the documentation you have prepared. This step is crucial for avoiding delays.
Attach Required Documents: Compile your submission package, which should include the completed form and all necessary supporting documents. If you are applying for multiple products, ensure you have invoices showing the sale of each product, as this is required for compliance. Double-check for completeness to prevent any issues during processing.
Submit the Form: Depending on the authority, you can submit your form online, by mail, or in person. Adhere to the specific submission guidelines provided by the agency to ensure compliance. Consider utilizing platforms such as Swiftdox, which simplify the submission process for requests related to the certificate of free sale.
Pay Fees: Be prepared to cover any applicable fees linked to the process of applying. For instance, the cost for a certificate of free sale can vary, with member rates typically around $60 and non-member rates at $80. Specific costs for different regions include $350 for Indonesia and $250 for Kuwait. Keep a receipt for your records as proof of payment.
Track Your Submission: After submission, monitor the status of your request. Some agencies offer tracking services, while others may require you to follow up directly. Processing times can vary, with general processing taking 5-7 business days and expedited options available for an additional fee, allowing for completion in as little as 1-2 business days. Additionally, be aware that some certificates may require apostille and notarization for compliance with foreign governing agencies.
By following these steps, you can navigate the CFS procedure more effectively, ensuring compliance with international trade requirements and facilitating smoother market access for your products.
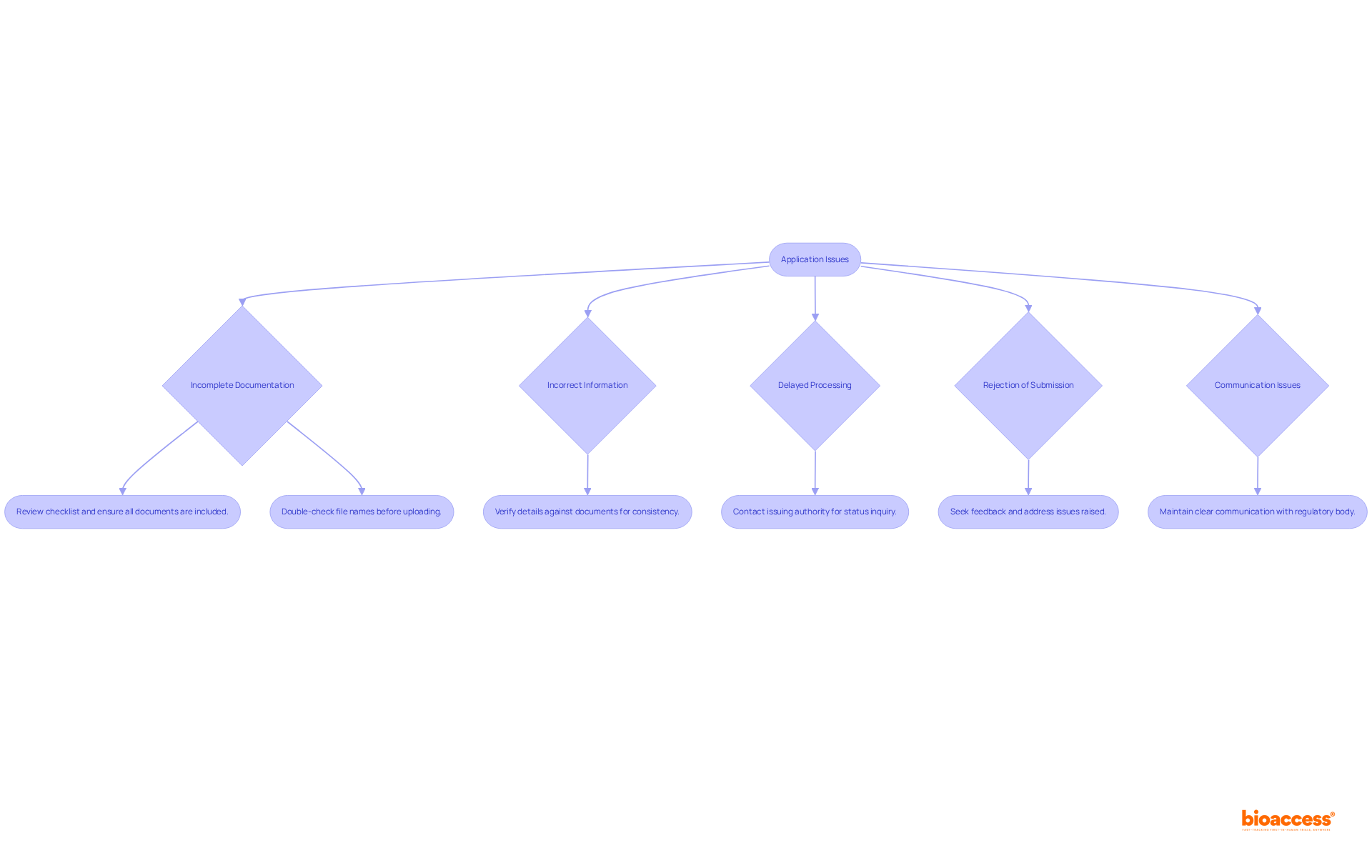
Even with meticulous preparation, problems can arise during the procedure for obtaining a certificate of free sale. Understanding these challenges is crucial for ensuring a smooth application process for obtaining a certificate of free sale. Here are some common issues and effective troubleshooting strategies:
Incomplete Documentation: If your submission is returned due to missing documents, review the checklist and ensure all required items are included. Additionally, double-check that files are named clearly and correctly before uploading to the SUGAM portal to avoid confusion. Resubmit promptly to facilitate processing.
Incorrect Information: Should you receive a notice about incorrect information, verify all details against your documents. Ensure consistency in product names and specifics across all documents to prevent discrepancies. Correct any issues and resubmit to uphold the integrity of your application.
Delayed Processing: If your request is taking longer than anticipated, contact the issuing authority to inquire about the status. Be prepared to provide your reference number. Remember, the speed of completion largely depends on the completeness and accuracy of your request.
Rejection of Submission: In the event your submission is denied, seek feedback from the authority to comprehend the reasons. Address the issues raised and provide complete, satisfactory explanations based on the information requested. Consider reapplying with the corrected information to enhance your chances of approval.
Communication Issues: Maintain clear communication with the regulatory body. If you encounter difficulties, do not hesitate to ask for clarification or assistance. Ensure that queries are fully responded to with detailed explanations and any required additional documentation.
Statistics indicate that incomplete documentation is a significant cause of application delays, with many applications facing rejection due to minor omissions. By ensuring thoroughness and clarity in your submissions, you can significantly reduce the risk of encountering these common pitfalls.
Securing a Certificate of Free Sale (CFS) is a pivotal step for companies aiming to penetrate international markets, particularly within the regulated sectors of medical devices and pharmaceuticals. This document not only verifies that a product is legally sold in its country of origin but also acts as a crucial assurance to foreign authorities regarding compliance with safety and efficacy standards. Understanding the significance of the CFS and the process to obtain it is vital for businesses aspiring to enhance their credibility and facilitate smoother customs clearance.
The article delineates a thorough approach to acquiring a CFS, commencing with an understanding of the document's importance and the specific requirements for various regions. It underscores the necessity of assembling the appropriate documentation, including:
Moreover, the step-by-step submission process is articulated, emphasizing the importance of accuracy and completeness in applications to avert common pitfalls such as:
In conclusion, while the journey to obtaining a Certificate of Free Sale may appear intricate, it can be navigated successfully with meticulous preparation and attention to detail. Companies are encouraged to leverage expert guidance and resources to streamline their applications and enhance their market access. By prioritizing compliance and grasping the nuances of the CFS, businesses can position themselves advantageously in the global marketplace, ultimately fostering growth and trust with international partners.
What is a certificate of free sale (CFS)?
A certificate of free sale (CFS) is an official document issued by a government authority that certifies an item is legally sold in its country of origin and complies with local regulations.
Why is a certificate of free sale important for exporting?
The CFS is essential for exporting medical devices, pharmaceuticals, and other regulated items, as it assures foreign authorities that these products meet safety and efficacy standards.
How does a certificate of free sale facilitate international market entry?
The CFS helps facilitate smoother customs clearance and fosters trust with foreign regulators and customers, making it crucial for companies aiming to penetrate international markets.
In which regions is the certificate of free sale particularly important?
The certificate of free sale is particularly important in regions such as Latin America and the Balkans, where it often serves as a prerequisite for market entry.
Can you give an example of a country that requires a certificate of free sale?
In India, the certificate of free sale is required for medical device registration, highlighting its significance in global trade.
What should companies do to ensure compliance with certificate of free sale requirements?
Companies should familiarize themselves with the specific requirements of the regions they are targeting, as these can vary significantly, and consult with experts for guidance.
How does the certificate of free sale enhance the credibility of medical devices?
The CFS serves as a guarantee from the importing nation to the exporting nation regarding the authenticity of the manufacturer and its products, enhancing credibility in global markets.
What strategic advantage can companies gain by utilizing the certificate of free sale?
Companies that effectively navigate the complexities of the healthcare sector and obtain their CFS can expedite their entry into lucrative markets and ensure compliance, positioning themselves advantageously in competitive landscapes.