


The article presents a comprehensive comparison of Electronic Data Capture (EDC) systems, examining their features, challenges, and future trends within the context of clinical trials. It underscores the substantial advantages of EDC platforms, including enhanced data integrity and expedited trial processes.
Simultaneously, it addresses the challenges associated with EDC, such as high initial costs and the necessity for extensive user training.
Ultimately, the article emphasizes the transformative potential of EDC technologies in modern medical research, highlighting their crucial role in advancing clinical practices.
The rapid evolution of Electronic Data Capture (EDC) systems is fundamentally reshaping the landscape of clinical trials, presenting a digital alternative to traditional data collection methods. These innovative platforms not only enhance data integrity and compliance but also promise to streamline trial processes, rendering them indispensable for researchers in the Medtech and Biopharma sectors. However, as the industry embraces these advancements, several challenges and complexities emerge that warrant careful consideration.
How can organizations effectively navigate the benefits and drawbacks of EDC systems to ensure successful implementation and maximize their potential in future research endeavors?
Electronic Data Capture (EDC) platforms are sophisticated digital solutions that significantly enhance the gathering, management, and storage of information produced during research trials. Unlike traditional paper methods, EDC platforms facilitate instantaneous information entry and oversight, thereby greatly improving the effectiveness and precision of research involving human subjects. By 2025, it is projected that approximately 70% of clinical trials will utilize EDC technologies, underscoring their growing acceptance within the industry.
These frameworks are crucial in ensuring data integrity and compliance with regulatory standards, offering immediate access to essential information. By minimizing manual data handling, EDC solutions mitigate the risk of transcription errors and enhance data quality, which is vital for reliable research outcomes. For instance, the implementation of EDC platforms such as MILO EDC has been shown to reduce the time required to develop studies by up to 50%, thereby accelerating the overall trial process.
EDC tools serve as versatile resources applicable throughout various stages of research trials, from early-phase studies to extensive experiments. Their ability to seamlessly integrate with other technologies, such as electronic health records (EHRs) and laboratory information management systems (LIMPs), further emphasizes their importance in modern research methodologies. This integration not only boosts operational efficiency but also ensures compliance with stringent data protection regulations, safeguarding that all collected information is securely stored and accessible solely to authorized personnel.
In conclusion, EDC platforms are transforming the research landscape by delivering unparalleled efficiency, precision, and insights, making them indispensable for Medtech, Biopharma, and Radiopharma innovators striving to advance medical technologies.
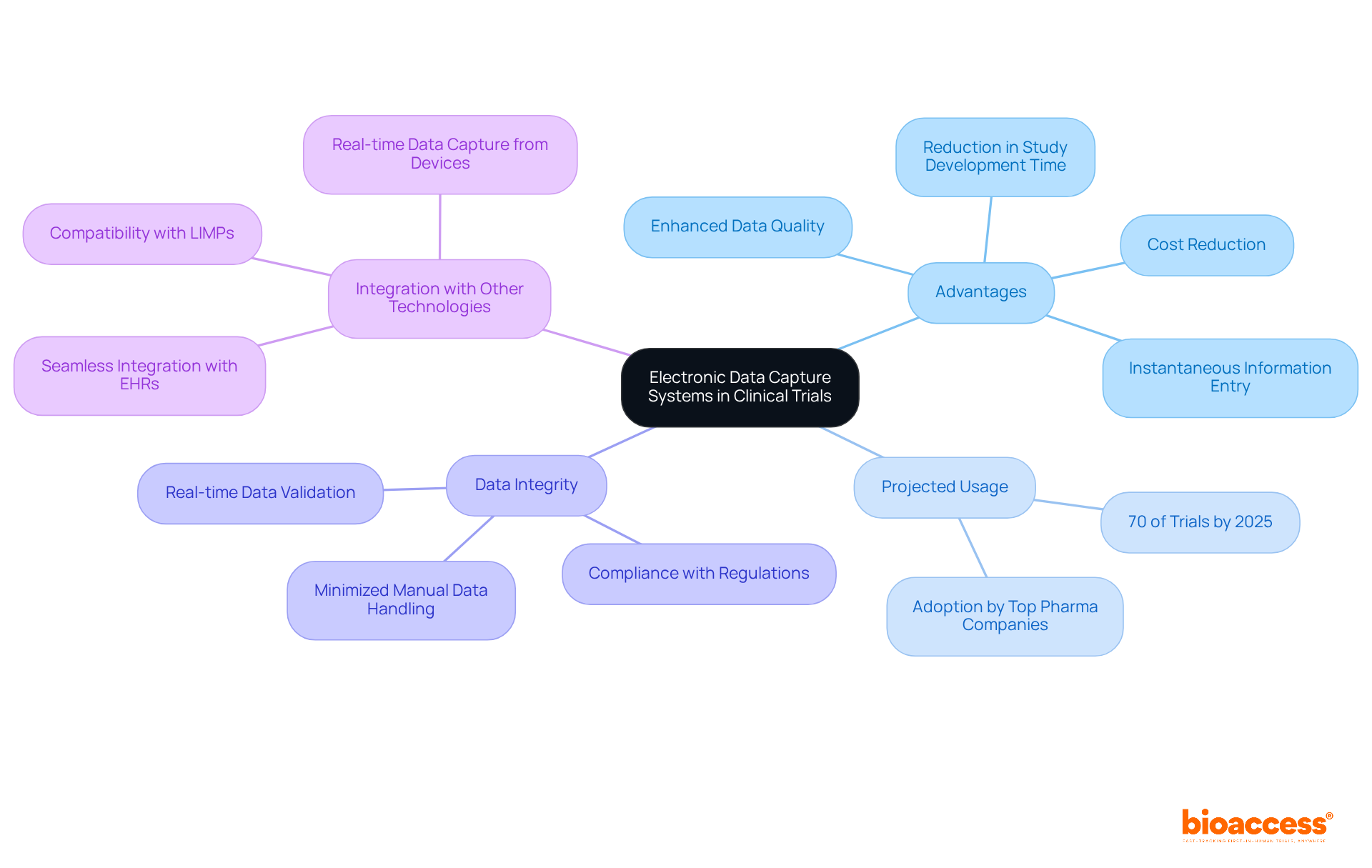
The EDC system comparison highlights that EDC solutions present a comprehensive array of features that significantly enhance the clinical trial process, particularly through the functionalities of bioaccess®. The key benefits include:
Together, these characteristics elevate information quality, accelerate trial timelines, and reinforce regulatory compliance, highlighting the importance of EDC system comparison as essential instruments in contemporary medical research. The incorporation of safety features exemplifies how these frameworks can streamline workflows and enhance information integrity, ultimately facilitating quicker market access for new therapies. As Deepu Joseph, Global Head of DM at Excelya, articulates, "In today's intricate trial landscape, interoperability and streamlined data flow are crucial." This statement underscores the pivotal role of EDC frameworks in enabling effective clinical research.

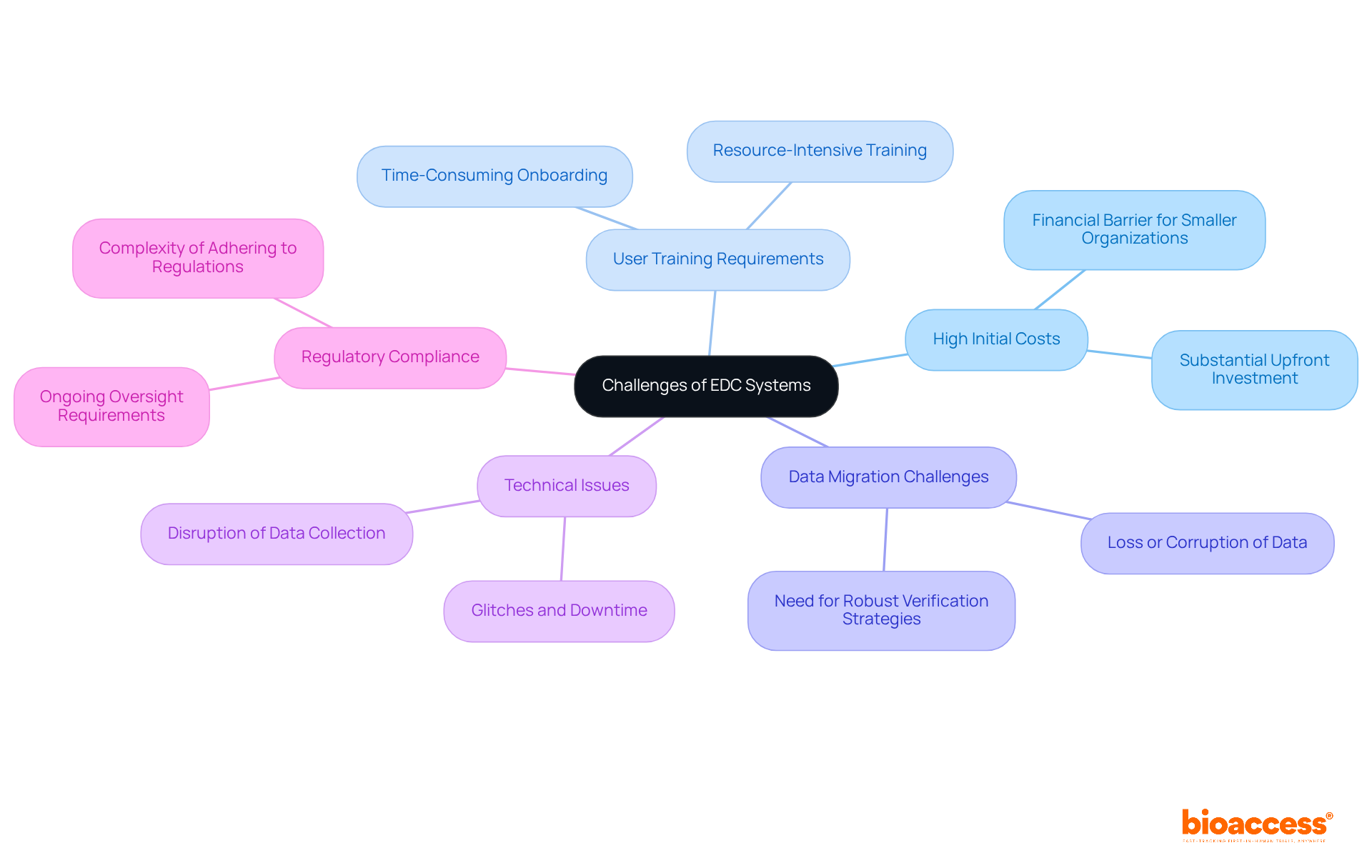
Despite their numerous advantages, Electronic Data Capture (EDC) platforms present several challenges that organizations must navigate effectively. Key drawbacks include:
High initial costs, which often require a substantial upfront investment. This financial barrier can be particularly significant for smaller organizations, as initial costs may encompass software licensing, hardware, and infrastructure upgrades, potentially reaching hundreds of thousands of dollars. Such financial burdens can hinder smaller organizations from embracing these frameworks, thereby limiting their ability to compete effectively in the clinical research landscape.
Effective utilization of EDC platforms necessitates comprehensive training for users. This training can be time-consuming and resource-intensive, with studies indicating that organizations may need to allocate several weeks for adequate user onboarding. Such demands can strain smaller teams with limited resources. As highlighted by external sources, the typical training duration required for EDC users can last several weeks, underscoring the necessity for organizations to prepare accordingly.
Shifting from conventional paper-based methods or outdated information management platforms to EDC can result in significant information migration challenges, including loss or corruption of data. A web survey involving 259 Canadian trials underscored the prevalence of these challenges, emphasizing the need for robust verification strategies to safeguard data integrity during this critical phase.
Technical issues also pose a risk, as EDC platforms are not immune to glitches or downtime, which can disrupt data collection and extend trial timelines. Organizations must be prepared for potential disruptions and have contingency plans in place to mitigate their impact on ongoing studies.
Ensuring that EDC platforms adhere to all regulatory requirements can be complex. Organizations must maintain ongoing oversight to navigate the evolving landscape of compliance, which adds to the operational burden. As Dr. Raoul Concepcion noted, "Maintaining GxP standards is crucial for the successful advancement of medical products."
In conclusion, these challenges necessitate thorough evaluation and strategic planning when selecting and implementing an EDC framework in clinical trials, particularly for smaller organizations seeking to leverage the advantages of digital information management.
The future of Electronic Data Capture (EDC) frameworks is poised for significant transformation, propelled by technological advancements and evolving regulatory landscapes. Key trends that will shape this evolution include:
Artificial Intelligence and Machine Learning: These technologies are set to enhance data analysis capabilities, enabling predictive analytics and improved decision-making. A study indicates that AI can boost enrollment in research trials by approximately 15% and reduce development timelines by about six months, showcasing its potential impact on EDC systems.
Mobile EDC Solutions: The rise of mobile technology is likely to yield more user-friendly EDC applications, facilitating input and monitoring on-the-go. Statistics suggest that mobile EDC solutions are expected to grow substantially, driven by the demand for adaptability and immediate information collection.
Blockchain Technology: The implementation of blockchain could significantly enhance information security and integrity, providing a tamper-proof record of entries. This technology fosters transparency and trust, which are essential in clinical research.
Patient-Centric Approaches: Future EDC frameworks may increasingly prioritize patient involvement, integrating tools that allow patients to input information directly, thereby improving accuracy and relevance. This shift empowers patients, transforming them into active participants in their healthcare journey.
Interoperability: As the healthcare ecosystem evolves, EDC platforms must ensure seamless integration with other digital health solutions, enhancing data flow and accessibility. This interoperability will be crucial for creating cohesive and efficient medical research processes.
In addition to these technological advancements, comprehensive trial management services—including feasibility studies, site selection, compliance assessments, trial setup, import permits, project oversight, and reporting—will play a vital role in the successful execution of these EDC platforms. The impact of medtech research studies on local economies—through job creation, economic growth, healthcare enhancement, and international collaboration—further underscores the importance of these advancements. These trends indicate a movement towards more efficient, secure, and user-friendly systems, as reflected in the EDC system comparison, ultimately enhancing research and patient outcomes. Expert predictions suggest that the integration of AI and mobile solutions will redefine the landscape of clinical trials, making them more adaptive and responsive to patient needs.

In conclusion, Electronic Data Capture (EDC) systems are fundamentally transforming clinical trials, delivering superior efficiency, accuracy, and compliance when compared to traditional data collection methods. As the industry transitions into a digital era, the anticipated surge in EDC technology adoption underscores its vital contribution to advancing research methodologies and enhancing patient outcomes.
This article has delved into the key features of EDC systems, such as:
These functionalities not only streamline trial processes but also uphold the integrity and security of data, effectively addressing the challenges associated with manual data management. Nonetheless, it is crucial to recognize the hurdles organizations may encounter during implementation, including significant initial investments and the necessity for extensive training, which may impede smaller entities from fully capitalizing on the benefits of EDC.
Looking forward, the trajectory of EDC systems will be influenced by innovations like:
These advancements are poised to bolster data security, enhance user experience, and promote patient-centric strategies within clinical trials. As the landscape of medical research evolves, adopting these technologies will be essential for organizations striving to maintain competitiveness and efficacy. The integration of EDC systems not only fosters operational efficiency but also lays the groundwork for more adaptable and responsive clinical research environments, ultimately serving to benefit patients and the healthcare sector at large.
What are Electronic Data Capture (EDC) systems?
EDC systems are sophisticated digital solutions that enhance the gathering, management, and storage of information produced during clinical research trials.
How do EDC platforms improve clinical trials compared to traditional methods?
EDC platforms facilitate instantaneous information entry and oversight, improving the effectiveness and precision of research involving human subjects, while minimizing manual data handling and reducing the risk of transcription errors.
What is the projected usage of EDC technologies in clinical trials by 2025?
It is projected that approximately 70% of clinical trials will utilize EDC technologies by 2025.
Why are EDC platforms important for data integrity and compliance?
EDC platforms ensure data integrity and compliance with regulatory standards by providing immediate access to essential information and securely storing data, which is accessible only to authorized personnel.
How do EDC systems impact the time required to develop studies?
The implementation of EDC platforms, such as MILO EDC, has been shown to reduce the time required to develop studies by up to 50%, accelerating the overall trial process.
At what stages of research trials can EDC tools be applied?
EDC tools can be applied throughout various stages of research trials, from early-phase studies to extensive experiments.
What technologies can EDC platforms integrate with?
EDC platforms can seamlessly integrate with other technologies such as electronic health records (EHRs) and laboratory information management systems (LIMPs).
How do EDC platforms contribute to operational efficiency?
The integration of EDC platforms with other technologies boosts operational efficiency and ensures compliance with stringent data protection regulations.
What industries benefit from the use of EDC platforms?
EDC platforms are indispensable for Medtech, Biopharma, and Radiopharma innovators striving to advance medical technologies.