


The article emphasizes the effective implementation of Corrective Action and Preventive Action (CAPA) in clinical trials, underscoring their critical role in addressing nonconformities and enhancing research quality. It presents a structured step-by-step process for CAPA implementation, highlighting the necessity for:
These elements are essential to ensure compliance and elevate patient safety, ultimately resulting in improved trial outcomes.
Understanding the nuances of corrective action and preventive action (CAPA) is crucial for enhancing the integrity of clinical trials. These processes not only address existing issues but also proactively mitigate potential risks, ensuring patient safety and data accuracy.
However, the path to effective CAPA implementation is often fraught with challenges, including resistance to change and resource constraints.
How can organizations navigate these hurdles to foster a culture of continuous improvement and ultimately elevate their research outcomes?
This exploration into CAPA is not merely an academic exercise; it is a vital step toward ensuring the success and reliability of clinical research.
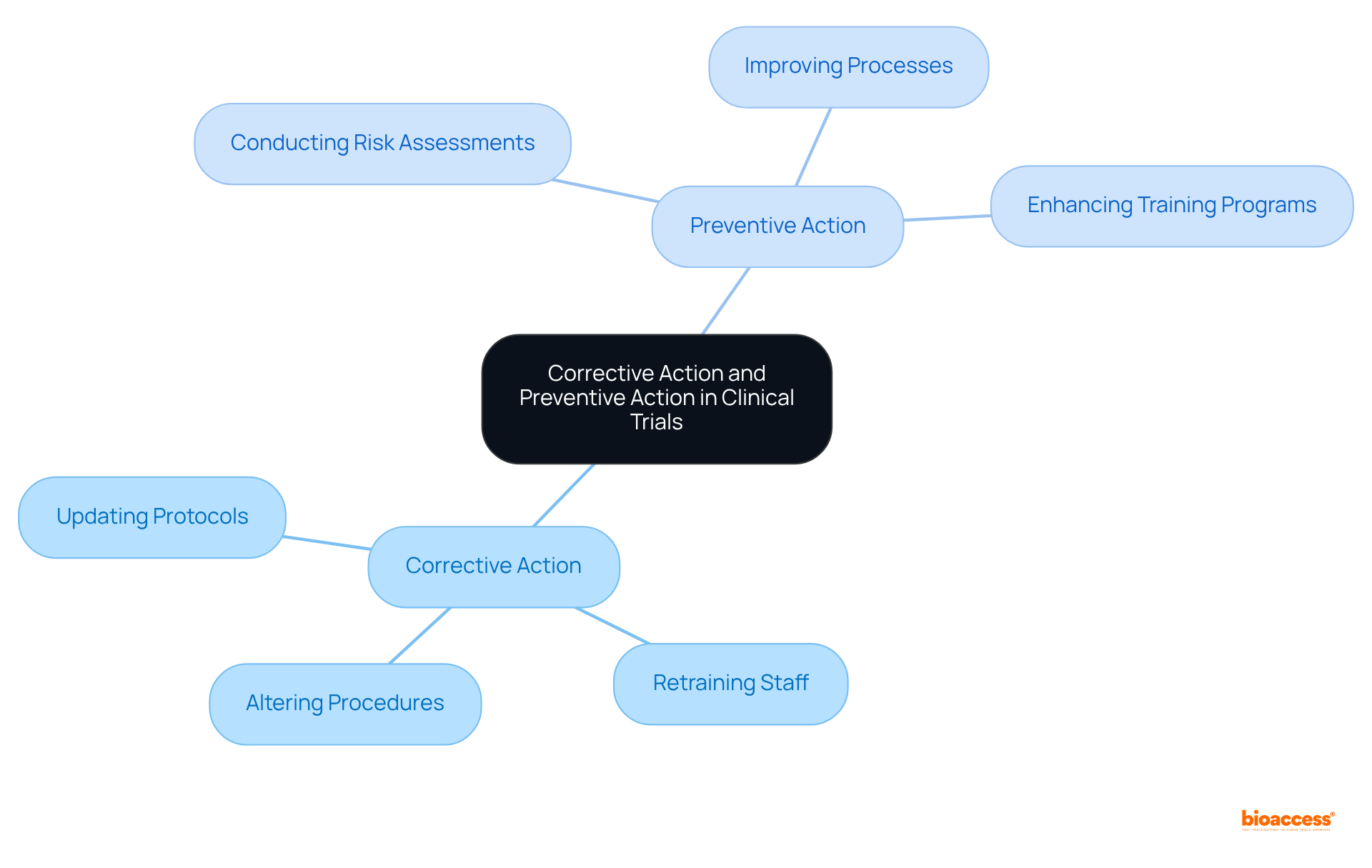
Corrective action and preventive action involve implementing measures to eliminate the root causes of existing nonconformities or undesirable situations in clinical trials. This may include:
In contrast, the focus of corrective action and preventive action is on proactive strategies aimed at eliminating potential causes of nonconformities before they occur. This can encompass:
Understanding these definitions is vital for ensuring compliance and enhancing the quality of research outcomes through corrective action and preventive action. Notably, entities that have successfully incorporated corrective action and preventive action procedures have observed a decrease of over 30% in nonconformities, underscoring the tangible benefits of these measures in healthcare environments.
The significance of corrective action and preventive action in medical research cannot be overstated. These processes are essential for swiftly addressing issues, thereby minimizing risks to patient safety and preserving data integrity. By adhering to regulatory requirements, organizations enhance their chances of obtaining trial approvals. The effective implementation of corrective action and preventive action cultivates a culture of continuous improvement, resulting in heightened operational efficiency and superior research outcomes.
At bioaccess, we offer comprehensive clinical trial management services, including:
All designed to facilitate efficient corrective action and preventive action methods. Thorough documentation and employee training are critical components of effective systems, ensuring that personnel are equipped to identify issues and implement remedial actions. Organizations that rigorously apply corrective action and preventive action methodologies have reported notable improvements in trial quality, with studies indicating that systematic approaches can reduce adverse events by as much as 30%.
This proactive stance enables organizations to detect and address both current and potential issues, ultimately enhancing patient safety and safeguarding the integrity of clinical data. Continuous monitoring and data analysis are vital to confirm the resolution of identified issues within the corrective action and preventive action framework.
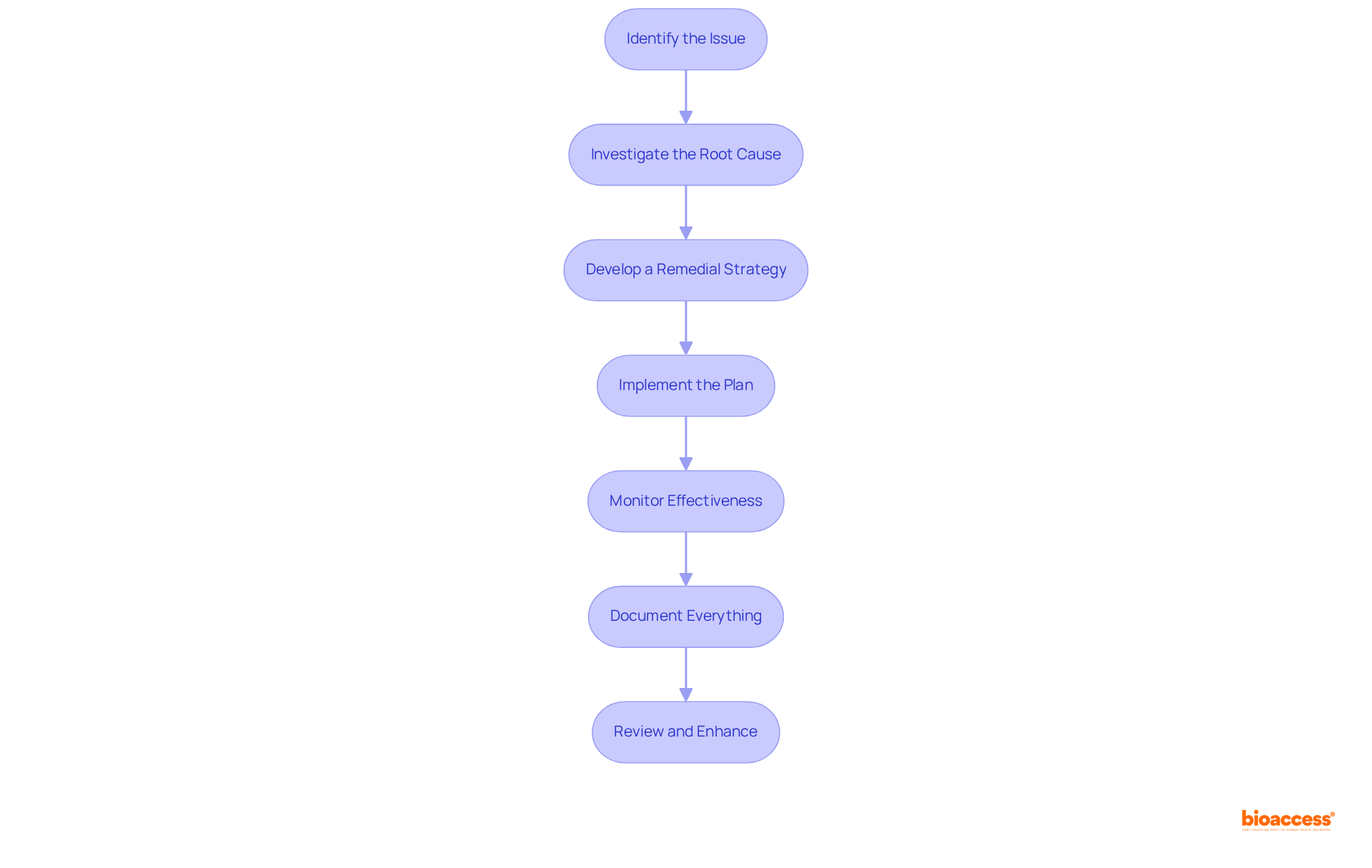
Identify the Issue: Begin by recognizing any nonconformities or potential risks that may arise from data discrepancies, protocol deviations, or adverse events. A proactive approach to identifying these issues is essential for the effective implementation of corrective action and preventive action (CAPA).
Investigate the Root Cause: Conduct a comprehensive investigation to uncover the underlying causes of the identified issues. While traditional tools like the 5 Whys or Fishbone Diagram can be useful, they may not always yield sufficient insights. Advanced methodologies, such as the TapRooT® Root Cause Analysis System, can enhance the depth of analysis and ensure that overlooked root causes are identified. Notably, studies have shown that 49% of root causes are system-related, while 46% are attributed to staff behavioral factors, underscoring the need for a thorough investigation.
Develop a Remedial Strategy: Create a comprehensive remedial strategy that outlines the measures necessary to tackle the identified issues. This plan should include clear timelines, designated responsible parties, and measurable outcomes to track progress effectively. It's important to note that 82% of recommendations from root cause analysis reports were rated weak, highlighting the necessity for a robust plan.
Implement the Plan: Carry out the corrective measures as detailed in the plan. It is crucial to ensure that all team members are informed and adequately trained on any new procedures or protocols to facilitate smooth implementation.
Monitor Effectiveness: After implementation, closely observe the results to verify the efficacy of the corrective measures. Gather pertinent information and responses to evaluate the effect, ensuring that the steps taken result in significant enhancements.
Document Everything: Keep comprehensive records of the complete corrective and preventive action, including issue identification, investigation findings, action plans, implementation steps, and monitoring results. This documentation is essential for regulatory compliance and serves as a reference for future corrective action and preventive action efforts.
Review and enhance the corrective action and preventive action process regularly to identify opportunities for improvement. This continuous assessment aids the organization in staying proactive in tackling potential problems and improves the overall efficiency of the corrective and preventive action system. As noted by experts, simple tools like the 5 Whys are often inadequate for effective root cause analysis, emphasizing the importance of adopting advanced systems like TapRooT®.
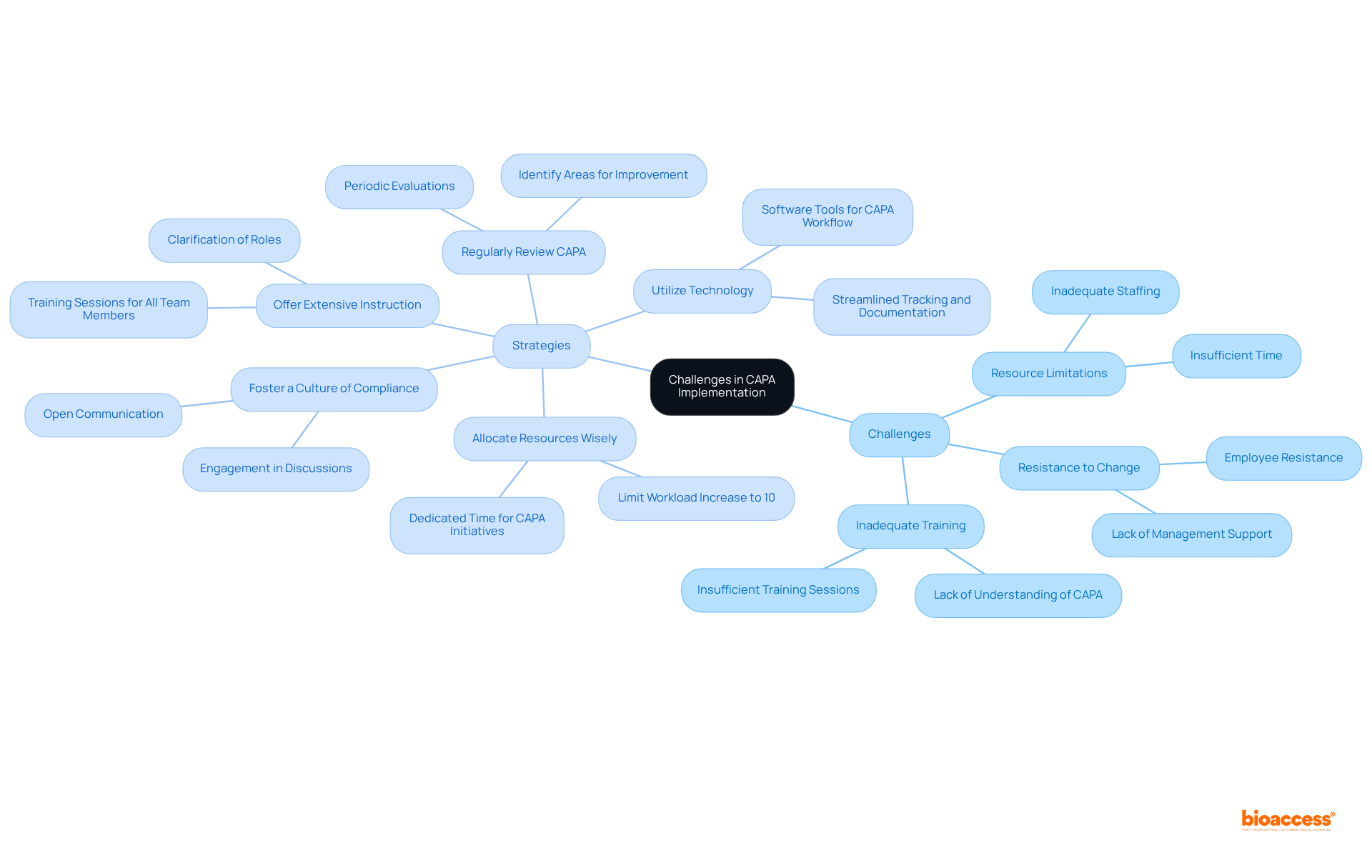
Implementing corrective action and preventive action can pose significant challenges, particularly resistance to change, resource limitations, and inadequate training. To effectively navigate these obstacles, consider the following strategies:
Foster a Culture of Compliance: Cultivating an environment that values open communication about the importance of corrective and preventive actions is crucial. Engaging team members in discussions about its benefits can enhance buy-in and reduce resistance. Statistics indicate that 29% of employees report that changes aren’t communicated clearly, highlighting the need for clear communication.
Allocate Resources Wisely: Dedicate adequate resources, including time and staff, to the corrective action and preventive action initiative. Focusing on corrective action and preventive action during project planning guarantees that these initiatives receive the attention they need. Companies should also be mindful not to increase employee workload by more than 10% during transformation to avoid resistance.
Offer Extensive Instruction: Conduct training sessions to prepare all team members with a clear grasp of the corrective action and preventive action and their specific roles. This knowledge can mitigate resistance and facilitate smoother implementation.
Utilize Technology: Employ software tools designed to simplify the corrective and preventive action workflow. These tools can streamline tracking issues, documenting actions, and monitoring effectiveness, making the task more manageable for everyone involved.
Regularly Review Corrective Action and Preventive Action: Conduct periodic evaluations of the corrective action and preventive action to identify areas for improvement. This practice ensures that the process remains effective and aligned with the organization’s evolving needs.
Statistics reveal that 70% of change projects fail due to employee resistance and lack of management support, and only 34% of change initiatives succeed, underscoring the necessity of robust planning and leadership alignment. By addressing these challenges head-on, organizations can enhance their efforts in corrective action and preventive action implementation and ultimately improve clinical trial outcomes. As Jack Welch wisely stated, "Change before you have to.
Implementing corrective action and preventive action (CAPA) is essential for enhancing the quality and integrity of clinical trials. These processes address existing nonconformities and proactively prevent future issues, ensuring compliance and improving research outcomes. By understanding and applying effective CAPA strategies, organizations can significantly reduce risks to patient safety and data integrity.
This article highlights critical steps for successful CAPA implementation, including:
It emphasizes the importance of thorough documentation and ongoing training to empower staff in recognizing and addressing potential problems. Furthermore, the discussion on overcoming challenges such as resistance to change and resource limitations provides valuable insights into fostering a culture of compliance and continuous improvement.
Ultimately, the successful implementation of CAPA is not merely a regulatory requirement; it is a commitment to excellence in clinical research. Organizations are encouraged to embrace these practices, as they enhance operational efficiency and contribute to safer and more reliable clinical trial outcomes. By prioritizing CAPA, stakeholders can ensure compliance standards are met while advancing the overall quality of healthcare research.
What are corrective actions in clinical trials?
Corrective actions in clinical trials involve implementing measures to eliminate the root causes of existing nonconformities or undesirable situations. This may include updating protocols, retraining staff, and altering procedures to prevent recurrence.
What are preventive actions in clinical trials?
Preventive actions focus on proactive strategies aimed at eliminating potential causes of nonconformities before they occur. This can encompass conducting risk assessments, improving processes, and enhancing training programs.
Why is it important to understand corrective action and preventive action in clinical trials?
Understanding these definitions is vital for ensuring compliance and enhancing the quality of research outcomes through corrective action and preventive action.
What impact do corrective action and preventive action have on nonconformities in clinical trials?
Entities that have successfully incorporated corrective action and preventive action procedures have observed a decrease of over 30% in nonconformities, highlighting the tangible benefits of these measures in healthcare environments.