


The article defines a Case Report Form (CRF) as an essential document in clinical research that systematically collects and organizes participant data, thereby enhancing data integrity and ensuring regulatory compliance.
Effective CRF management is underscored, involving:
These practices are critical to ensuring accurate and reliable data collection, ultimately supporting the success of clinical trials.
In the realm of clinical research, the Case Report Form (CRF) emerges as a cornerstone for data collection, meticulously crafted to capture essential information from study participants. Understanding the intricacies of CRFs not only enhances the reliability of trial outcomes but also ensures compliance with regulatory standards. As the shift from traditional paper forms to electronic formats gains momentum, researchers encounter the challenge of adapting to these advancements while maintaining data integrity.
What key steps can be taken to effectively implement and manage CRFs? How can these practices elevate the quality of clinical research?
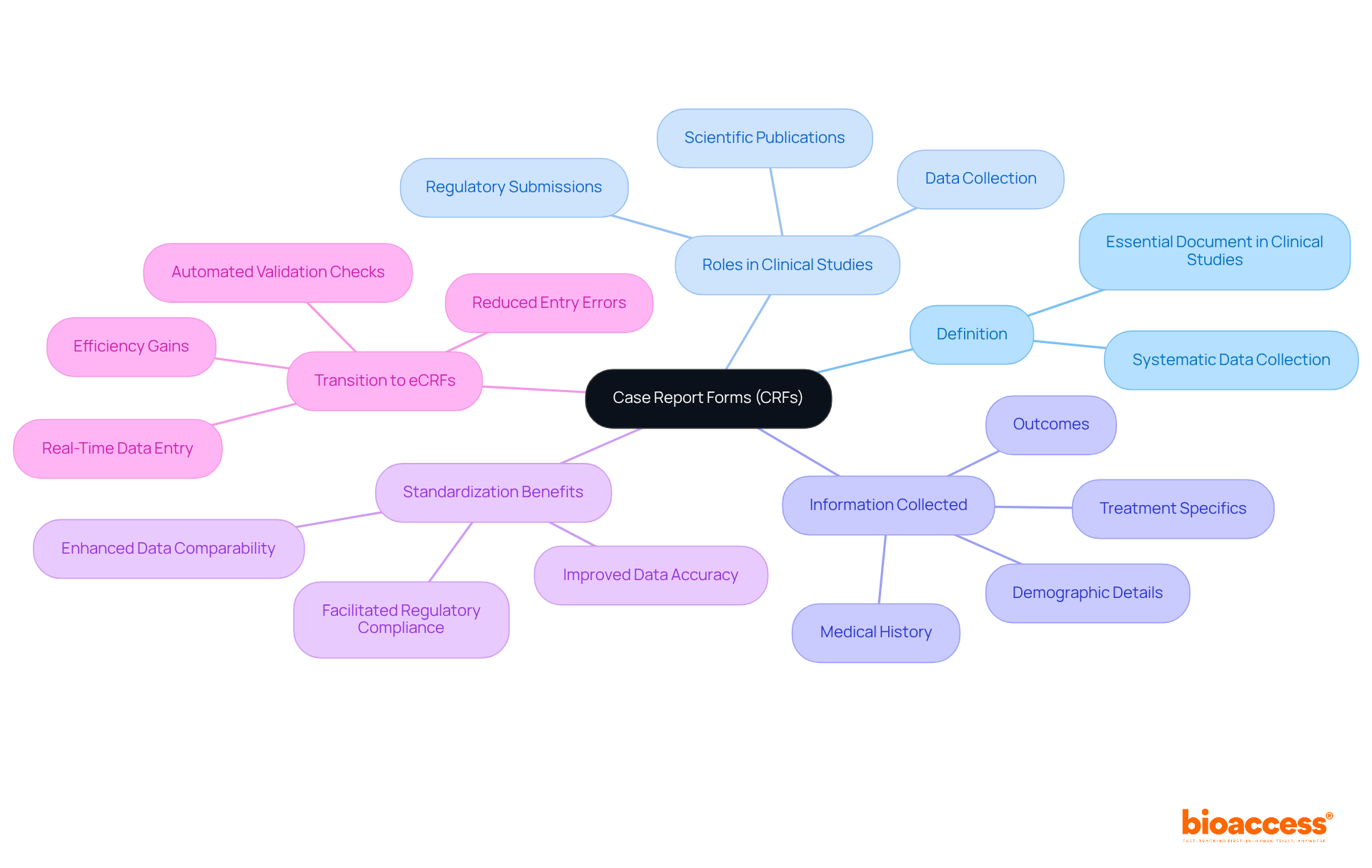
To understand the role of a Case Report Form (CRF), we must define CRF as an essential document in clinical studies, meticulously designed to collect organized information from each participant. Its primary role is to capture all relevant information required by the study protocol, encompassing demographic details, medical history, treatment specifics, and outcomes.
By standardizing the collection of information, case report forms simplify the analysis and reporting of trial outcomes while also playing a vital role in regulatory submissions and scientific publications. Notably, the transition from traditional paper-based CRFs to electronic Case Report Forms (eCRFs) has significantly improved information accuracy and reduced entry errors, with eCRFs achieving a 0% error rate compared to a 5% error rate for paper forms. This advancement enhances information integrity, ensuring that the gathered details are both comprehensive and reliable.
As emphasized, 'To define CRF, a clinical study case report form is created to gather systematic and consistent information from each participating patient to facilitate precise analysis and regulatory submissions.' Understanding how to define CRF is crucial for researchers, as this knowledge underscores their importance in upholding information integrity and adhering to ethical standards in clinical research.
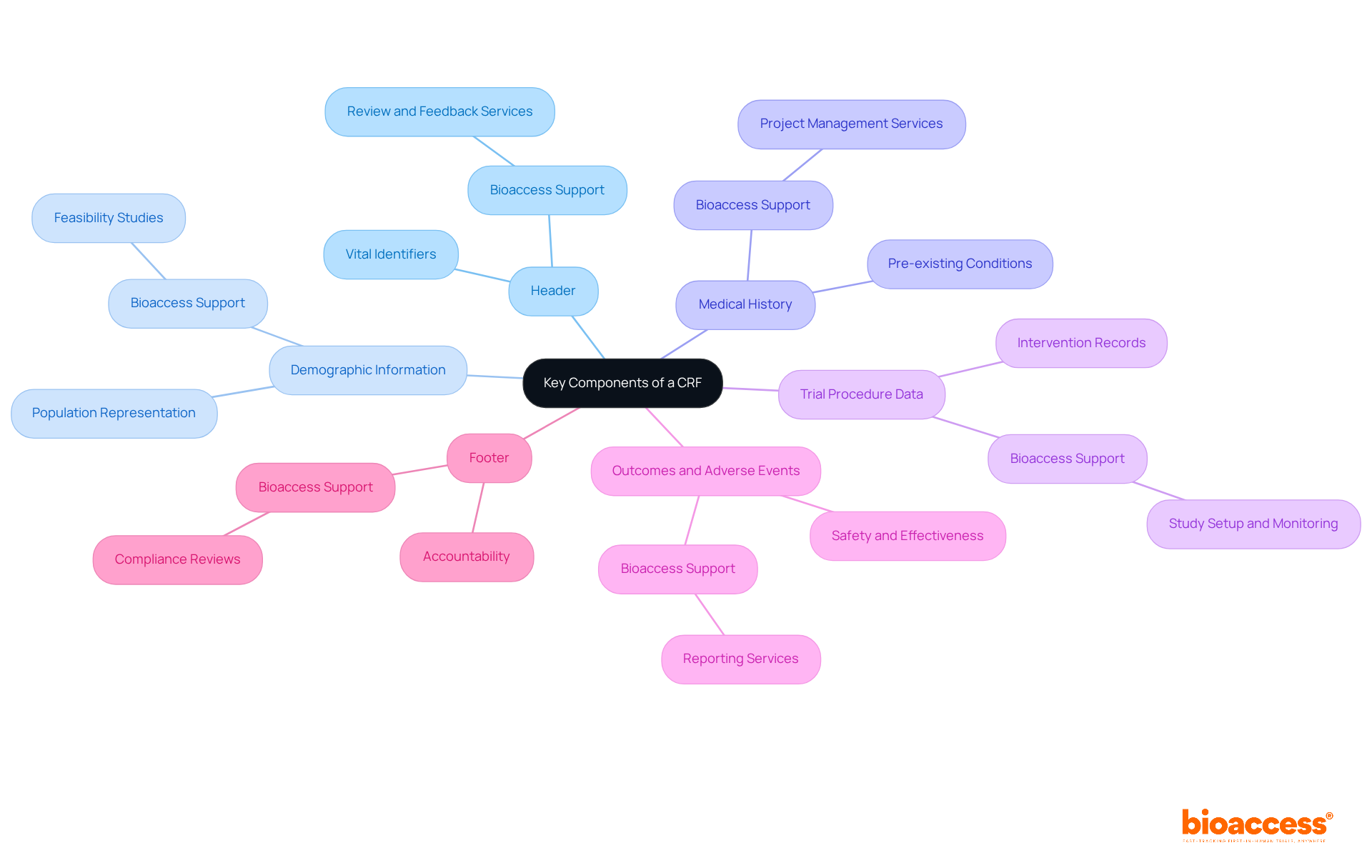
A well-structured Case Report Form (CRF) encompasses several essential components that are critical to the success of clinical trials:
Integrating these elements enables researchers to define CRF that enhance the precision and effectiveness of data gathering. This ultimately aids the success of clinical trials, especially with the extensive services offered by Bioaccess.
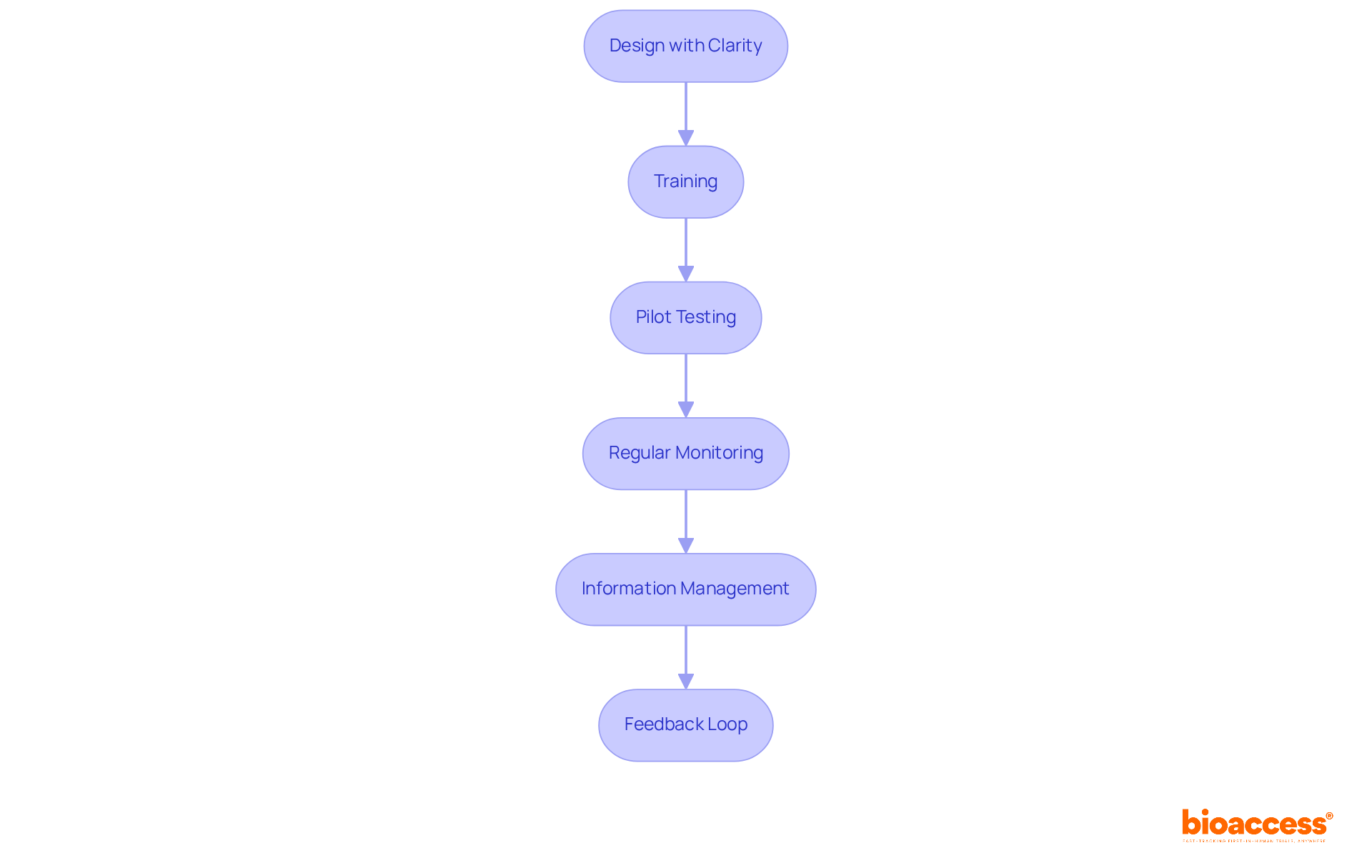
To effectively implement and manage Case Report Forms (CRFs) in clinical research, it is essential to define CRF and follow a series of strategic steps.
Design with Clarity: Begin by creating user-friendly case report forms that feature clear instructions and a logical flow. Avoid jargon and vague terms to minimize confusion during information entry, which is critical for precise data collection. User-friendly CRFs help to define CRF by significantly reducing mistakes and inaccuracies in entry, enhancing the overall quality of the research.
Training: Provide comprehensive training for all personnel involved in information collection. This training should define CRF, covering the layout, information entry procedures, and the importance of accurate reporting, ensuring that staff are well-prepared to manage the forms effectively. Robust training programs are vital for maximizing the potential of clinical trials.
Pilot Testing: Conduct a pilot test to define CRF with a small group of participants, in order to identify potential issues or areas for improvement before full-scale implementation. This step is crucial for refining the CRF to define CRF based on real user experiences, aligning with best practices for CRF design.
Regular Monitoring: Establish a system for continuous oversight of information entry to define CRF requirements and ensure adherence. This system may include regular audits and feedback sessions with information gatherers, which are essential for maintaining data integrity. Regular monitoring guarantees that the information collected is both precise and trustworthy.
Information Management: Whenever possible, utilize electronic CRFs (eCRFs), which help to define CRF by offering numerous benefits such as real-time information validation, easier retrieval, and enhanced security. eCRFs have proven to reduce entry mistakes and improve overall quality, making them a preferred choice among clinical researchers.
Feedback Loop: Implement a feedback mechanism that allows users to report challenges or suggest improvements to the CRF. Continuous enhancement is necessary for maintaining the efficiency of the information-gathering process, as user-focused design can significantly improve usability and engagement. Incorporating iterative testing and feedback from actual users will enhance case report forms based on their experiences.
By adhering to these steps, researchers can define CRF to ensure effective implementation and management, leading to high-quality data collection and successful study outcomes.
Defining a Case Report Form (CRF) is pivotal in clinical research, serving as the backbone for collecting and organizing critical data from participants. This essential document ensures a standardized approach to data collection, enhancing the accuracy and integrity of the information gathered—elements crucial for regulatory compliance and scientific validation.
Key components contribute to an effective CRF, including:
Each of these elements fosters an environment where high-quality data can be collected and analyzed. The transition to electronic CRFs (eCRFs) marks a significant improvement in minimizing errors and streamlining the data management process, reinforcing the necessity of embracing technological advancements in clinical research.
Ultimately, the successful implementation and management of CRFs are essential for the integrity and efficacy of clinical trials. Researchers and clinical teams must prioritize best practices in CRF design and management, ensuring compliance with regulatory standards while contributing to the advancement of medical knowledge. By doing so, they can significantly enhance the quality of clinical research outcomes, paving the way for innovations that improve patient care and treatment efficacy.
What is a Case Report Form (CRF)?
A Case Report Form (CRF) is an essential document in clinical studies designed to collect organized information from each participant, including demographic details, medical history, treatment specifics, and outcomes.
What is the primary purpose of a CRF?
The primary purpose of a CRF is to capture all relevant information required by the study protocol, which simplifies the analysis and reporting of trial outcomes.
How do CRFs contribute to regulatory submissions and scientific publications?
CRFs play a vital role in regulatory submissions and scientific publications by standardizing the collection of information, which ensures that the data is comprehensive and reliable.
What are the advantages of electronic Case Report Forms (eCRFs) over traditional paper-based CRFs?
eCRFs have significantly improved information accuracy and reduced entry errors, achieving a 0% error rate compared to a 5% error rate for paper forms, which enhances information integrity.
Why is it important for researchers to understand CRFs?
Understanding CRFs is crucial for researchers as it underscores their importance in upholding information integrity and adhering to ethical standards in clinical research.