


The article delves into the significant advances and challenges associated with utilizing stem cells for the treatment of Type 1 Diabetes (T1D), underscoring their potential to regenerate insulin-producing beta cells and enhance patient outcomes. It is crucial to acknowledge that, despite recent clinical trials yielding promising results, there are substantial hurdles to overcome. These include:
Addressing these challenges is essential to fully harness the benefits of stem cell therapies in the management of T1D.
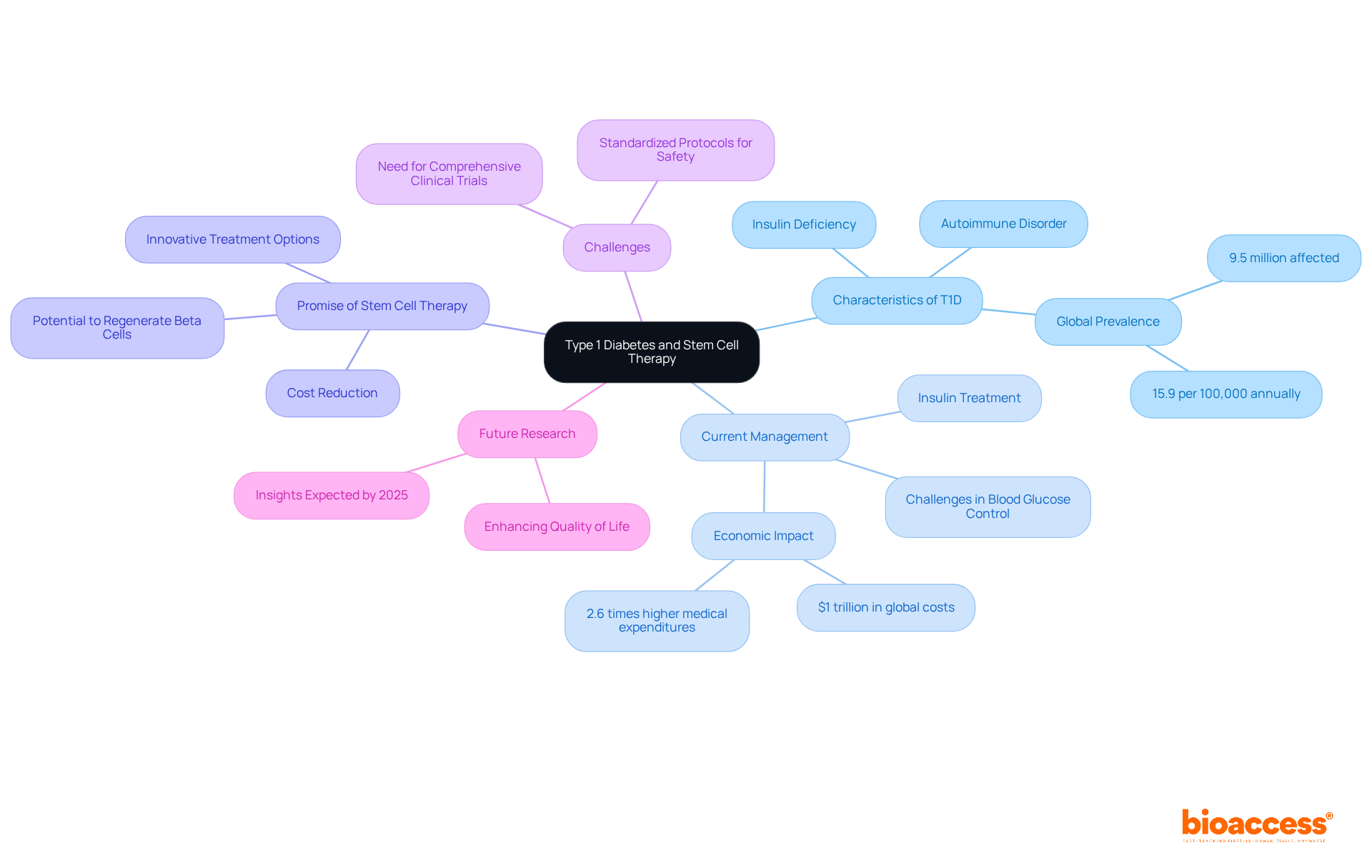
Type 1 Diabetes (T1D) presents a formidable challenge for millions globally, as the immune system relentlessly targets the insulin-producing beta cells in the pancreas. Current management strategies often fall short, making the exploration of innovative treatments increasingly critical. This article investigates the promising realm of stem cell therapy, emphasizing its potential to regenerate beta cells and restore natural insulin production. Yet, amid the excitement surrounding recent breakthroughs, significant hurdles persist. What will it take to surmount these challenges and fully harness the transformative power of stem cell therapies for T1D?
Type 1 Diabetes (T1D) represents a significant challenge in the realm of autoimmune disorders, characterized by the immune system's relentless attack on insulin-producing beta cells within the pancreas, leading to a critical insulin deficiency. Currently, this ongoing condition affects approximately 9.5 million individuals worldwide, necessitating lifelong management through insulin treatment. Despite notable advancements in diabetes care, a considerable number of patients struggle to achieve optimal blood glucose control, which highlights the urgent necessity for innovative treatment options.
Stem progenitor therapy utilizing diabetes type 1 stem cells has emerged as a promising avenue for addressing T1D, with the potential to regenerate functional beta structures and restore natural insulin production. Recent studies indicate that precursor tissues can evolve into insulin-producing units, potentially offering a cure for diabetes type 1 stem cells instead of merely managing the condition. This innovative approach could substantially reduce the burden on healthcare systems, which are grappling with soaring costs associated with diabetes management—currently estimated at over $1 trillion globally. By leveraging bioaccess®, we can expedite clinical trials, addressing the pressing need for effective solutions while simultaneously curtailing costs.
Experts in the field underscore the transformative possibilities that diabetes type 1 stem cells in tissue therapy present. As one researcher noted, advancements in this domain could lead to breakthroughs that fundamentally reshape the care landscape for T1D. Nevertheless, challenges persist, including the necessity for more comprehensive clinical trials and the establishment of standardized protocols to ensure safety and efficacy.
Looking ahead to 2025, ongoing research and clinical trials are expected to yield further insights into the efficacy of regenerative treatments for T1D, potentially paving the way for new therapeutic alternatives that could significantly enhance the quality of life for millions affected by this condition. As the global prevalence of T1D continues to rise, innovative solutions such as bioaccess® and stem therapy are essential in addressing the healthcare challenges posed by this chronic condition.
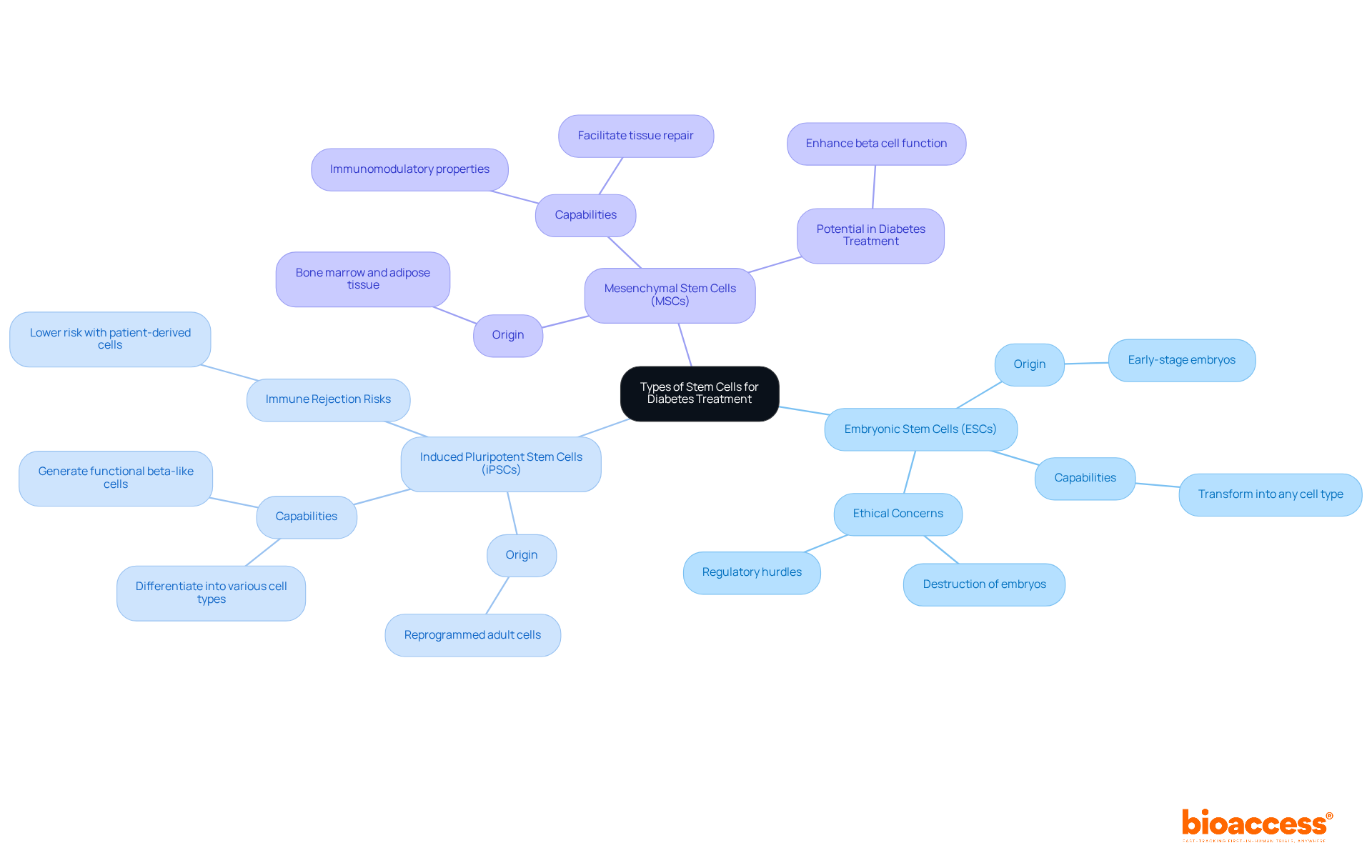
Stem cells can be categorized into several types based on their origin and differentiation potential, which is crucial for advancing clinical research in regenerative medicine.
Embryonic Stem Cells (ESCs): Originating from early-stage embryos, these cells possess the remarkable capability to transform into any type of cell, including insulin-producing beta cells. However, their application is constrained by ethical concerns and regulatory hurdles, particularly regarding the destruction of embryos, which raises significant moral questions.
Induced Pluripotent Stem Cells (iPSCs): These adult cells are reprogrammed to an embryonic-like state, enabling them to differentiate into various types of cells. iPSCs present a promising alternative to ESCs, as they can be derived from the patient’s own tissues, significantly lowering the risk of immune rejection. Recent advancements in diabetes type 1 stem cells have illuminated their potential in generating functional beta-like cells, which could revolutionize the treatment of diabetes.
Mesenchymal Stem Cells (MSCs): Found in a variety of tissues, including bone marrow and adipose tissue, MSCs exhibit immunomodulatory properties and can facilitate tissue repair. They are currently under investigation for their ability to enhance beta cell function and longevity in diabetic individuals, representing a complementary approach to conventional treatments.
Understanding these progenitor types is essential for developing effective therapies with diabetes type 1 stem cells. As research continues to explore their applications and address the ethical concerns associated with their use, collaboration among researchers, clinicians, and ethicists will be vital in navigating the complexities of stem cell research.
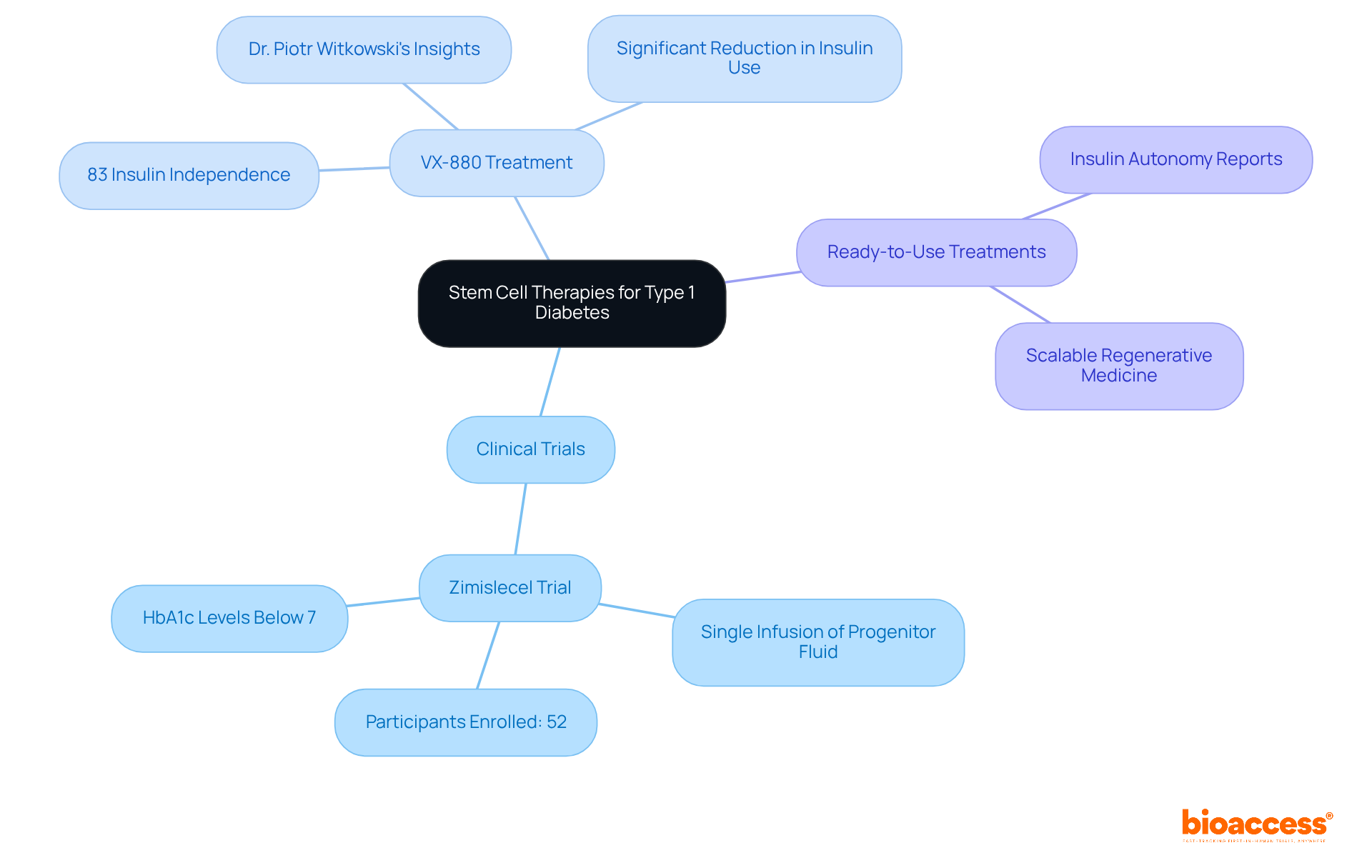
Recent advancements in therapies using diabetes type 1 stem cells have demonstrated significant promise in clinical settings.
Clinical Trials: Ongoing trials are investigating the efficacy of stem cell-derived islet cells. Notably, the Zimislecel trial, which includes a single infusion of progenitor fluid, is presently recruiting participants for FDA authorization. Initial findings suggest that participants receiving cell infusions have shown substantial enhancements in insulin production and glycemic control, with many achieving HbA1c levels below 7%.
VX-880 Treatment: Developed by Vertex Pharmaceuticals, VX-880 is an innovative cell-derived treatment that has shown promise in restoring insulin independence. Initial findings from clinical studies indicate that 83% of participants attained insulin independence after one year, underscoring the treatment's capacity to significantly reduce or eliminate the need for insulin injections. Dr. Piotr Witkowski emphasized that these findings warrant further evaluation of VX-880, which could transform T1D care.
Ready-to-Use Treatments: Investigational approaches utilizing ready-to-use progenitor entities are also yielding favorable outcomes. Some patients have reported achieving insulin autonomy following treatment, suggesting a shift towards more accessible and scalable regenerative medicine.
These advancements illustrate the transformative potential of regenerative medicine in managing diabetes type 1 stem cells, paving the way for a more curative approach to treatment.
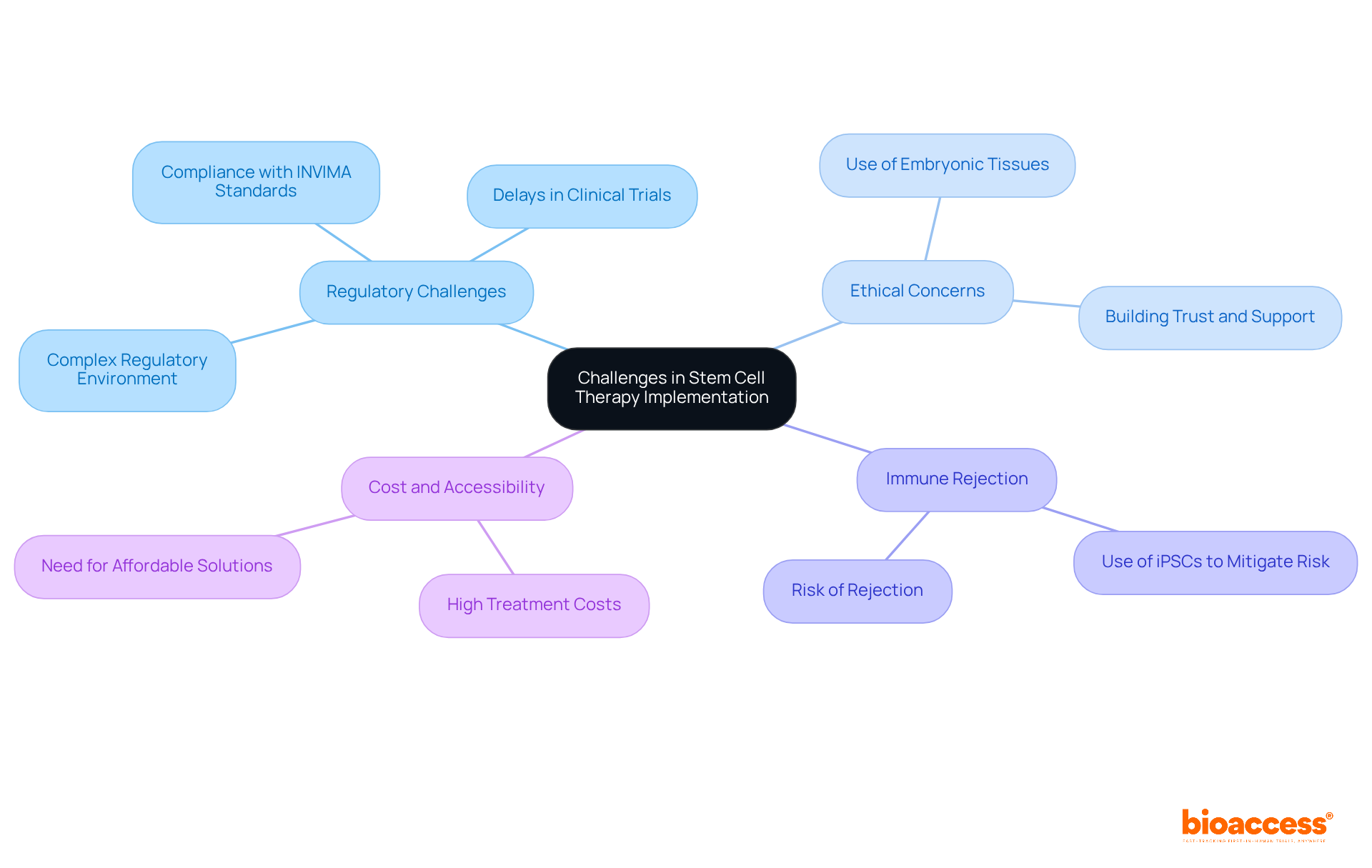
Despite the promising potential of stem cell therapies for Type 1 Diabetes, several challenges persist:
Regulatory Challenges: The regulatory environment for regenerative treatments is complex and varies significantly across regions. In Colombia, the INVIMA (Colombia National Food and Drug Surveillance Institute) plays a crucial role in overseeing the marketing and manufacturing of health products, including medical devices. As a Level 4 health authority recognized by the Pan American Health Organization/World Health Organization, INVIMA ensures compliance with safety, efficacy, and quality standards. Navigating these regulations can lead to delays in clinical trials and the approval process for new treatments, impacting timely access to innovative therapies. As noted, the worldwide tissue market is expected to attain USD 36.97 billion by 2030, underscoring the increasing interest and investment in this field.
Ethical Concerns: The utilization of embryonic precursor tissues raises profound ethical questions that can hinder research advancement and societal acceptance. Adhering to strict ethical standards is crucial for building trust and support for this type of research.
Immune Rejection: A significant challenge is the risk of immune rejection of transplanted tissues. To mitigate this risk, strategies such as using induced pluripotent stem cells (iPSCs) obtained from the patient’s own tissues are being examined; however, these methods require further research to confirm their effectiveness and safety.
Cost and Accessibility: The elevated expenses associated with regenerative treatments can limit availability for numerous patients. Developing affordable solutions and ensuring equitable distribution of these interventions are essential for their broad acceptance and integration into standard care protocols.
Addressing these obstacles is vital for the successful implementation of diabetes type 1 stem cells in managing Type 1 Diabetes, paving the way for innovative and effective treatment options.
Additionally, bioaccess® offers comprehensive clinical trial management services, including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting. These capabilities are essential for navigating the regulatory landscape and accelerating the development of stem cell therapies.
The exploration of stem cell therapy for Type 1 Diabetes (T1D) presents a landscape rich with both promise and challenges. This innovative approach aims not only to manage T1D but to potentially cure it by regenerating insulin-producing beta cells. As advancements in stem cell research continue to unfold, the hope for a transformative treatment option intensifies, underscoring the significance of ongoing studies and clinical trials.
Recent developments reveal that various types of stem cells, including induced pluripotent stem cells and mesenchymal stem cells, possess considerable potential for restoring insulin production and enhancing patient outcomes. Clinical trials, exemplified by the promising VX-880 treatment, demonstrate the tangible benefits these therapies can offer, with numerous participants achieving insulin independence. Nonetheless, challenges such as regulatory complexities, ethical considerations, and cost barriers must be addressed to enable broader access to these groundbreaking therapies.
In light of these advancements, it is imperative for stakeholders—including researchers, healthcare providers, and policymakers—to collaborate in overcoming the obstacles that impede the implementation of stem cell therapies for T1D. By fostering an environment conducive to innovation and ethical research, the potential to revolutionize diabetes treatment is within reach, offering renewed hope to millions impacted by this chronic condition. Embracing these advancements could pave the way for a future where managing Type 1 Diabetes becomes a relic of the past.
What is Type 1 Diabetes (T1D)?
Type 1 Diabetes (T1D) is an autoimmune disorder where the immune system attacks insulin-producing beta cells in the pancreas, leading to a critical deficiency in insulin.
How many people are affected by T1D worldwide?
Approximately 9.5 million individuals worldwide are affected by Type 1 Diabetes.
What are the current management strategies for T1D?
The current management strategy for T1D involves lifelong insulin treatment, although many patients struggle to achieve optimal blood glucose control.
What is stem progenitor therapy and how does it relate to T1D?
Stem progenitor therapy involves using diabetes type 1 stem cells to regenerate functional beta structures in the pancreas, potentially restoring natural insulin production and offering a cure for T1D.
What potential benefits does stem cell therapy offer for T1D?
Stem cell therapy could reduce the burden of diabetes management on healthcare systems, which currently face costs exceeding $1 trillion globally, by providing a potential cure rather than just managing the condition.
What challenges exist in the development of stem cell therapy for T1D?
Challenges include the need for more comprehensive clinical trials and the establishment of standardized protocols to ensure the safety and efficacy of the treatments.
What is the significance of the bioaccess® platform in T1D research?
Bioaccess® is expected to expedite clinical trials for stem cell therapies, addressing the urgent need for effective solutions while helping to reduce costs.
What are the expectations for T1D research and clinical trials by 2025?
Ongoing research and clinical trials are expected to provide further insights into the efficacy of regenerative treatments for T1D, potentially leading to new therapeutic alternatives that could significantly improve the quality of life for those affected.