


Understanding the complexities of clinical trials is crucial for ensuring their success, particularly in Romania, where the role of Principal Investigators (PIs) has become increasingly vital. This article explores the essential training requirements that equip PIs with the skills necessary to navigate regulatory landscapes, prioritize patient safety, and uphold ethical standards in research.
As the demand for high-quality medical studies continues to grow, what challenges do PIs face in meeting these training standards? How can they ensure they remain at the forefront of clinical research excellence?
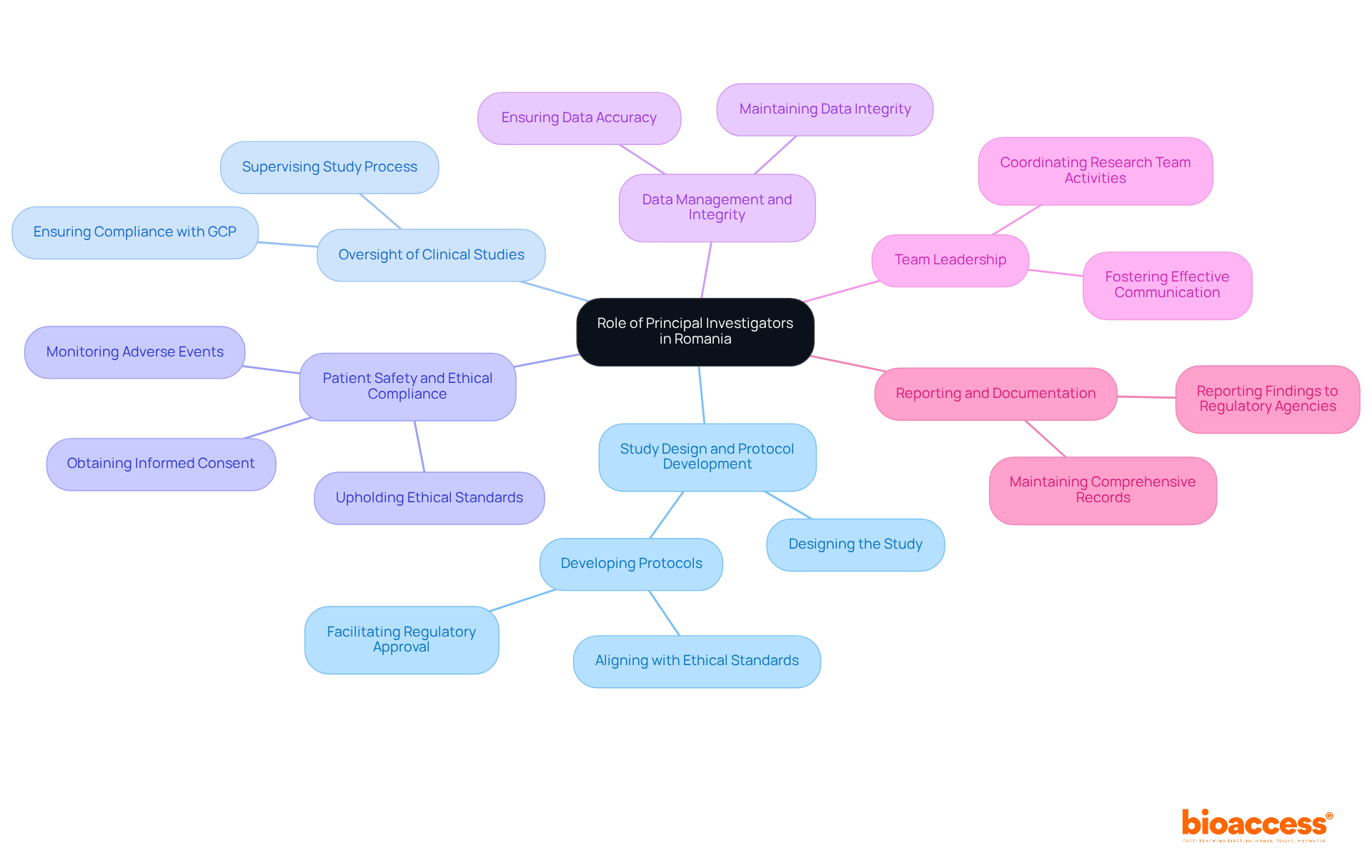
Principal Investigators (PIs) in Romania play a pivotal role in the successful execution of clinical trials, with their responsibilities spanning several critical areas:
The role of PIs is increasingly recognized as essential in the context of evolving regulatory environments and the growing complexity of research studies. Their ability to navigate these challenges directly influences the success of medical studies in Romania.
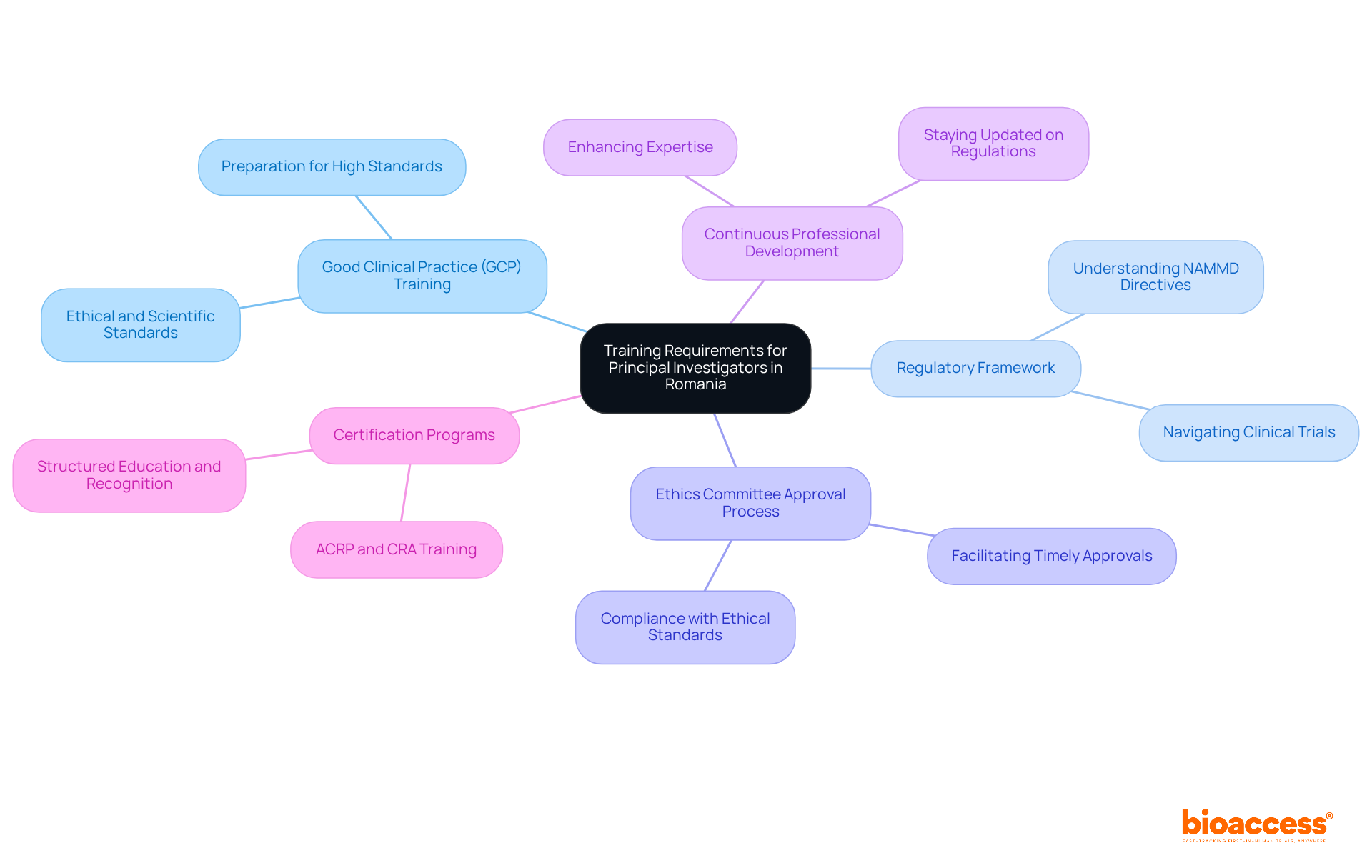
In Romania, Principal Investigators (PIs) must fulfill the training requirements for Romanian principal investigators to comply with national and EU regulations. This training is essential for meeting the training requirements for Romanian principal investigators, ensuring the integrity of clinical research and the safety of participants.
Good Clinical Practice (GCP) Training is a cornerstone requirement. To fulfill the training requirements for Romanian principal investigators, PIs are required to undergo GCP training, which covers the ethical and scientific quality standards necessary for designing, conducting, and reporting clinical trials. This training equips PIs with the principles that govern medical studies, ensuring they are well-prepared to uphold high standards.
Understanding the Regulatory Framework is equally crucial. PIs need a comprehensive grasp of the Romanian regulatory environment, particularly the directives established by the National Agency for Medicines and Medical Devices (NAMMD). This knowledge enables them to navigate the complexities of clinical trials effectively.
Moreover, the training requirements for Romanian principal investigators regarding the Ethics Committee Approval Process are vital. Familiarity with this process ensures that studies comply with ethical standards, facilitating smoother interactions with ethics committees and leading to timely approvals.
Continuous Professional Development is encouraged for PIs to meet the training requirements for Romanian principal investigators, ensuring they stay updated on regulatory changes and advancements in medical methodologies. This commitment to lifelong learning not only enhances their expertise but also ensures adaptability in a rapidly evolving field.
Participation in Certification Programs, such as those offered by the Association of Clinical Research Professionals (ACRP) or Clinical Research Associate (CRA) training, can significantly bolster a PI's qualifications. These programs provide structured education and recognition of skills in healthcare studies.
Statistics indicate that the training requirements for Romanian principal investigators are resulting in consistently rising completion rates for GCP training, reflecting a growing dedication to maintaining high standards in medical studies. According to bioaccess®, "Understanding these regulatory requirements is crucial for ensuring that studies adhere to legal and ethical standards." Expert opinions underscore the significance of GCP training as a fundamental component for ensuring the integrity and success of studies in the region.
In Romania, the training requirements for Romanian principal investigators include access to a range of training programs and resources designed to enhance their skills in medical studies. This is crucial for ensuring high standards in clinical research. Key offerings include:
The shift towards online training is particularly noteworthy, as it allows for greater flexibility and accessibility. Statistics indicate a rising enrollment in training programs for healthcare studies throughout Romania, which emphasizes the training requirements for Romanian principal investigators and the importance of competency-based education in this area. Furthermore, Romania's medical study market is projected to triple from €72 million in 2019 to over €210 million by 2026, emphasizing the increasing significance of medical investigations and the necessity for well-trained principal investigators. Specialists assert that online platforms not only enhance education but also prepare PIs to meet the evolving demands of medical studies, ultimately facilitating patient access to groundbreaking treatments.

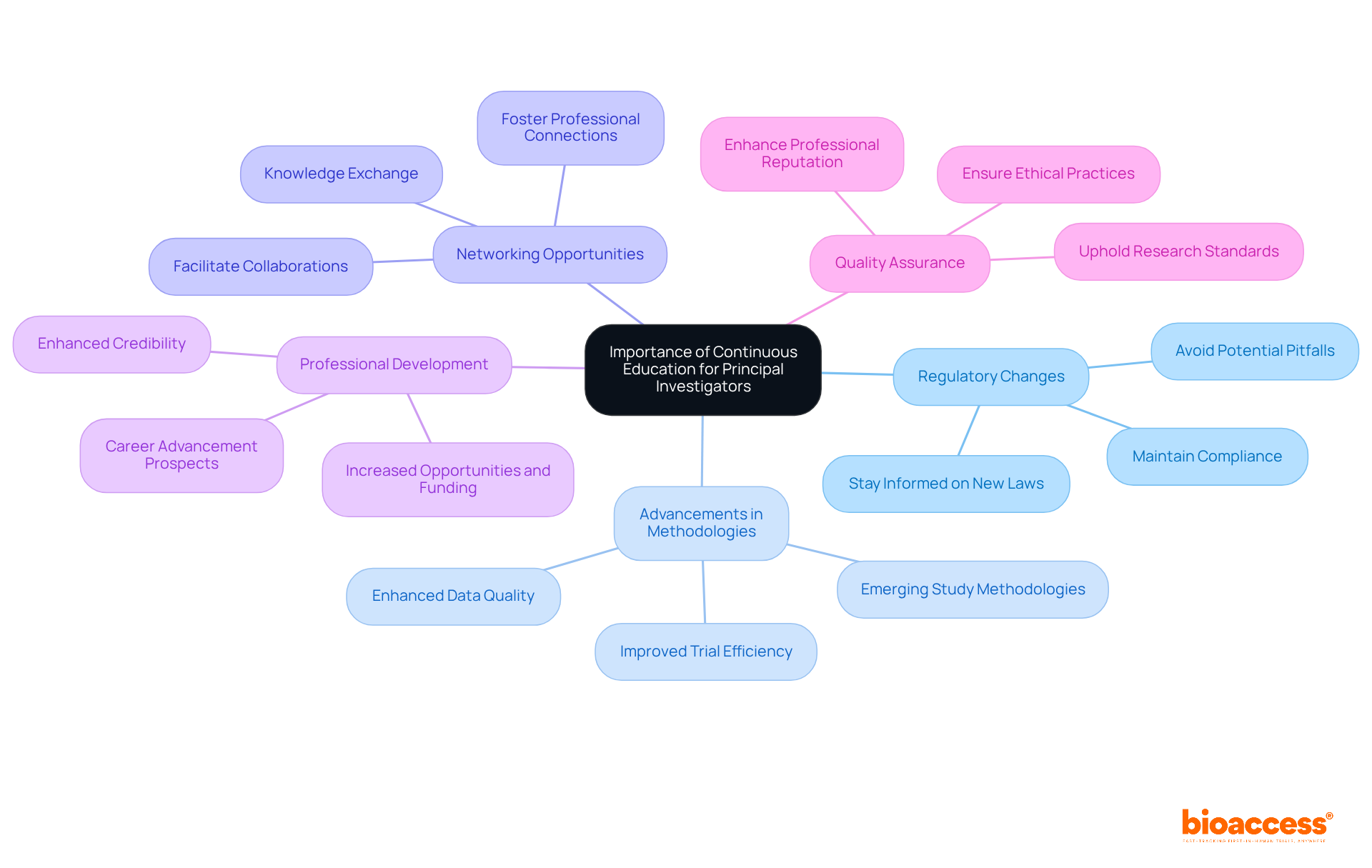
Ongoing education is essential for Principal Investigators (PIs) to fulfill the training requirements for Romanian principal investigators, enhancing their efficiency and ensuring compliance in medical studies. This necessity is underscored by several key factors:
Statistics reveal that continuous education can boost trial success rates by up to 30%, emphasizing its critical role in regulatory compliance and overall trial effectiveness. As the regulatory environment continues to evolve, PIs must prioritize ongoing education to fulfill the training requirements for Romanian principal investigators and successfully navigate these changes while maintaining their leadership in clinical research.
The pivotal role of Principal Investigators (PIs) in Romania is crucial, marked by their extensive responsibilities that include study design, patient safety, regulatory compliance, and team leadership. Their unwavering commitment to ethical standards and rigorous training not only bolsters the credibility of clinical trials but also protects the welfare of participants. Understanding the essential training requirements for Romanian principal investigators is vital for ensuring these professionals are well-equipped to navigate the complexities of clinical research effectively.
Key insights into specific training requirements, such as Good Clinical Practice (GCP) training, knowledge of the regulatory framework, and the importance of continuous education, have been emphasized throughout this article. The focus on professional development through various programs, workshops, and certification opportunities underscores the commitment to maintaining high standards in clinical research. This ongoing education is essential for PIs to adapt to regulatory changes and advancements in study methodologies, ultimately leading to improved trial outcomes and enhanced participant safety.
In conclusion, the importance of well-trained Principal Investigators cannot be overstated. As the landscape of clinical research evolves, PIs must prioritize their education and training to uphold the integrity of their studies and advance their careers. Investing in continuous professional development is not merely a personal benefit; it is a critical component of fostering a robust and ethical research environment in Romania. By embracing these training requirements and resources, Principal Investigators can ensure they remain at the forefront of medical research, significantly contributing to the advancement of healthcare and patient access to innovative treatments.
What is the role of Principal Investigators (PIs) in Romania?
Principal Investigators (PIs) in Romania are responsible for the successful execution of clinical trials, which includes study design, protocol development, oversight of clinical studies, ensuring patient safety, data management, team leadership, and reporting.
What are the responsibilities of PIs regarding study design and protocol development?
PIs are responsible for designing the study and developing the protocol, ensuring they align with ethical standards and scientific rigor, and facilitating regulatory approval while prioritizing participant safety.
How do PIs ensure oversight of clinical studies?
PIs supervise the entire clinical study process, ensuring compliance with regulatory requirements and Good Clinical Practice (GCP) guidelines, which is crucial for yielding reliable results and maintaining public trust.
What is the importance of patient safety and ethical compliance for PIs?
PIs prioritize patient safety by ensuring informed consent is obtained from all participants and that ethical standards are upheld throughout the study, protecting participant welfare and maintaining the study's integrity.
What is the PIs' role in data management and integrity?
PIs are accountable for the accuracy and integrity of the data collected during the study, as trustworthy data is essential for informed decisions regarding the safety and efficacy of investigational products.
How do PIs lead their research teams?
PIs coordinate activities and foster effective communication among team members and stakeholders, ensuring alignment with the study's objectives and regulatory requirements.
What are the reporting and documentation responsibilities of PIs?
PIs must maintain comprehensive records of the study, document adverse events and protocol deviations, and report findings to regulatory agencies and sponsors to ensure transparency and accountability.
Why is the role of PIs increasingly recognized in Romania?
The role of PIs is increasingly recognized due to evolving regulatory environments and the growing complexity of research studies, where their ability to navigate challenges directly influences the success of medical studies.