


In clinical research, the distinction between accuracy and precision is fundamental. Accuracy is defined as the closeness of a measurement to the true value, whereas precision refers to the consistency of repeated measurements, irrespective of their closeness to the true value. This article elucidates this difference with practical examples, such as blood pressure monitors, highlighting the critical role both accuracy and precision play in ensuring reliable research outcomes and safeguarding patient safety in clinical studies. Understanding these concepts is essential for researchers aiming to produce valid and trustworthy results.
Understanding the nuances between accuracy and precision is pivotal in the realm of clinical research, where the stakes are often life-altering.
This distinction is not merely academic; it has profound implications for patient safety and the validity of research findings.
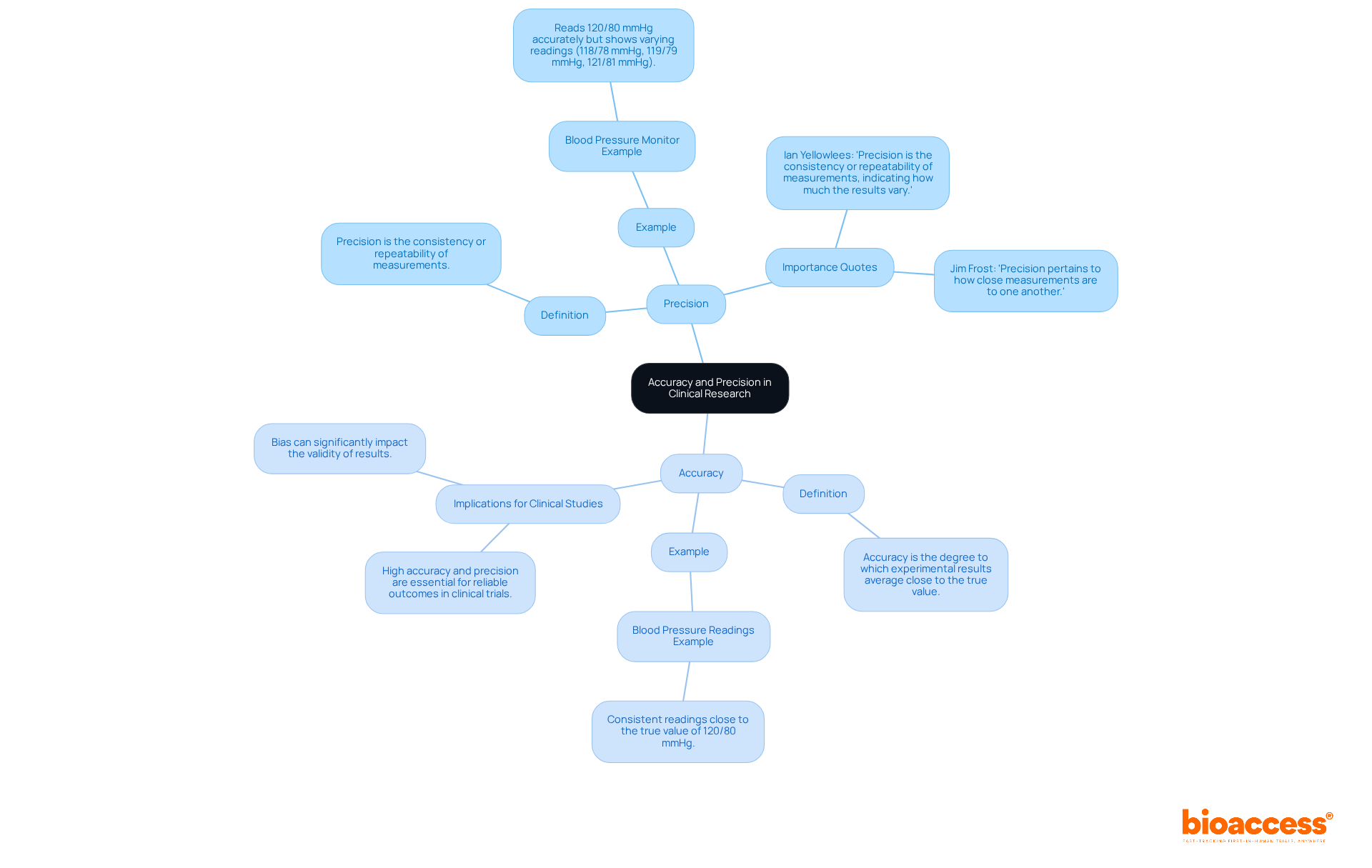
In clinical research, precision is defined as the extent to which a recorded value aligns with the genuine or accepted value, indicating the correctness of a calculation. A blood pressure monitor that reliably displays 120/80 mmHg when the true value is 120/80 mmHg illustrates precision.
Conversely, accuracy pertains to the consistency of repeated measurements, reflecting how closely these measurements cluster together, irrespective of their proximity to the true value. For instance, if the same blood pressure monitor provides readings of 118/78 mmHg, 119/79 mmHg, and 121/81 mmHg, it demonstrates consistency but may be inaccurate if the actual value remains at 120/80 mmHg.
To interpret information and findings in clinical studies effectively, it is essential to explain the difference between accuracy and precision, as high levels of both are crucial for dependable results and informed clinical decisions. As Ian Yellowlees states, "Precision is the consistency or repeatability of measurements, indicating how much the results vary."
Implementing standardized protocols and regular calibration can significantly enhance both accuracy and reliability, ultimately benefiting patient safety and advancing medical knowledge. Additionally, addressing bias is vital, as it can profoundly impact the validity of results, leading to erroneous conclusions.
What challenges do you face in achieving precision and accuracy in your clinical research endeavors?
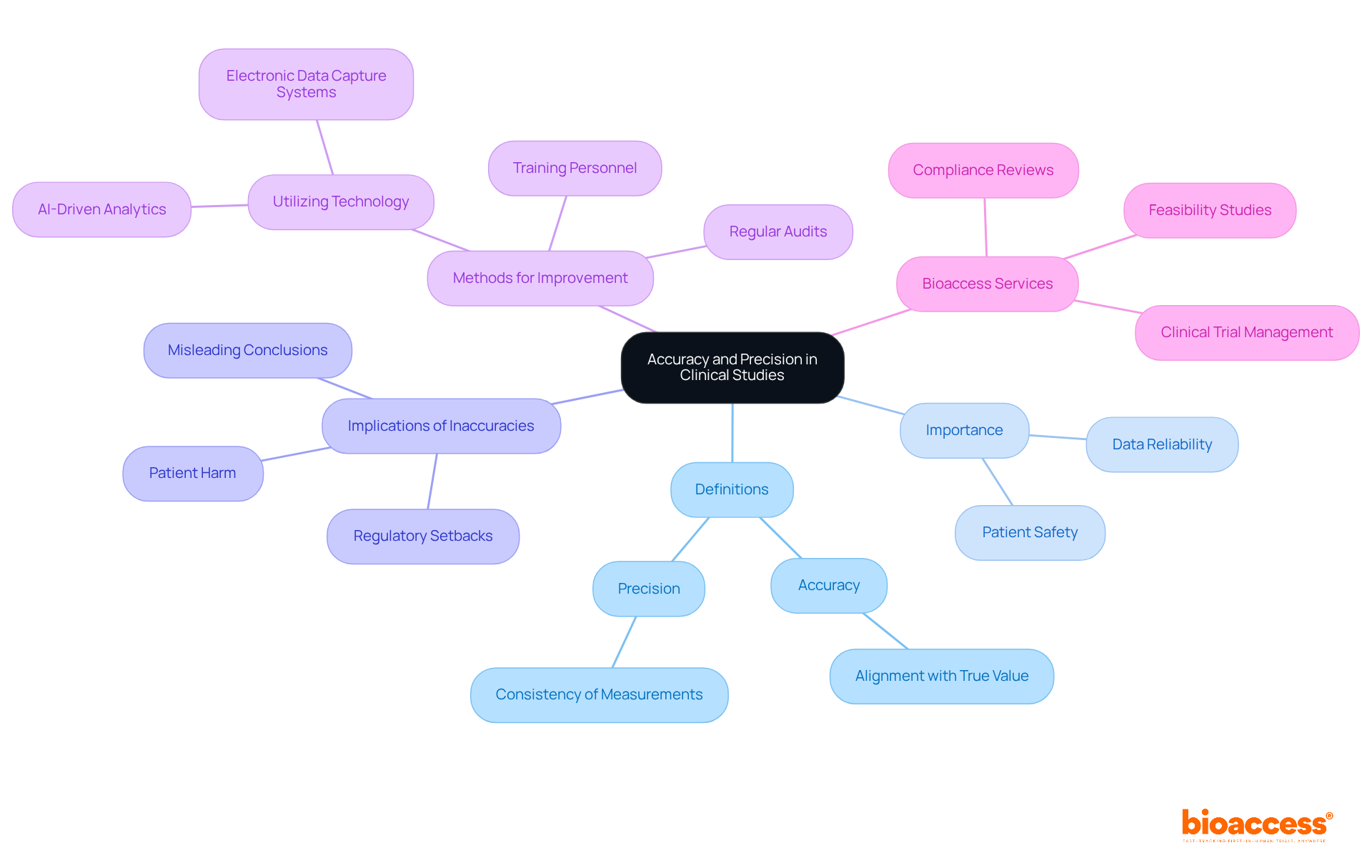
To ensure the integrity of clinical studies, it is essential to explain the difference between accuracy and precision, as both significantly influence the reliability of research findings. Precise evaluations reflect the true condition of the subjects under examination, which is crucial for making well-informed choices about treatment effectiveness and safety. Consider the implications: inaccuracies in data can lead to erroneous conclusions about a drug's effectiveness, potentially jeopardizing patient safety. In fact, approximately 30% of medical studies fail due to information-related issues, underscoring the necessity for meticulous data management.
Precision ensures that repeated measurements yield consistent results, essential for establishing data reliability. High accuracy is crucial to explain the difference between accuracy and precision, as it reduces variability in results and allows researchers to formulate more robust conclusions. For instance, employing electronic data capture systems can reduce data entry errors by up to 50%, thereby enhancing the overall quality of information collected. Regular audits and quality control assessments are also critical in upholding high standards of correctness and exactness in clinical studies.
At Bioaccess, our comprehensive clinical trial management services—ranging from feasibility studies and site selection to compliance reviews, trial setup, import permits, project management, and reporting—are designed to uphold the highest standards of accuracy and precision. Each service directly contributes to regulatory approvals and clinical guidelines, ultimately safeguarding individual welfare. As emphasized by the Bioaccess Content Team, prioritizing these standards is not merely a regulatory requirement but a moral obligation to protect patients and advance medical knowledge. Furthermore, comprehensive training for personnel involved in data collection is essential to ensure they are skilled in using assessment instruments and methods, thereby enhancing the reliability of clinical research.
To effectively measure and enhance accuracy and precision in clinical research, it is important to explain the difference between accuracy and precision and implement several key techniques.
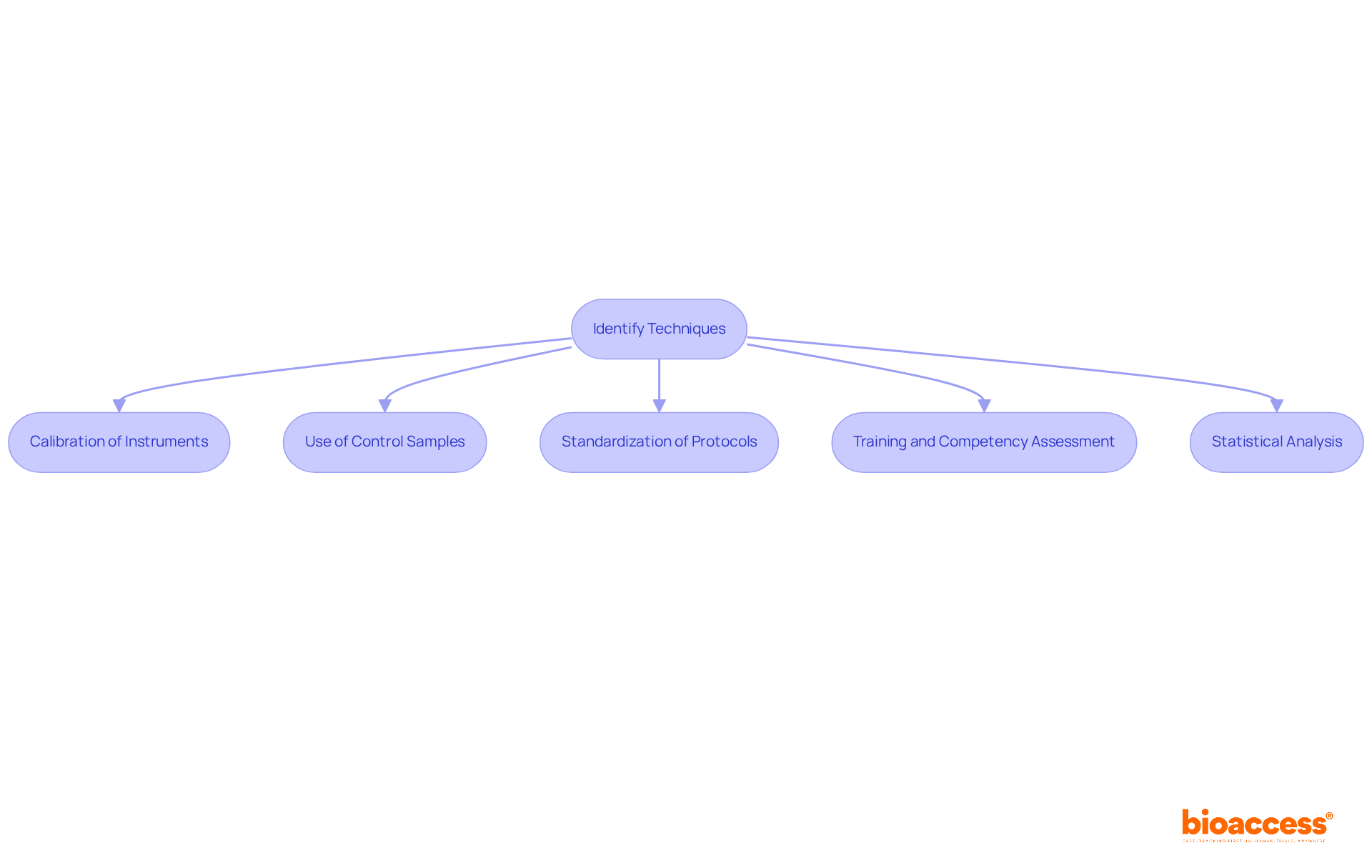
Calibration of Instruments: Regular calibration of testing devices against established standards is crucial. This process not only ensures accurate readings but also helps identify systematic errors that could compromise data integrity. Calibration should be conducted whenever changes are made to reagents or instruments, as this is essential for ensuring the reliability of results. As Tze Ping Loh aptly points out, "Calibration is an essential element for the reliability and correctness of mass spectrometry measurements."
Use of Control Samples: The incorporation of control samples in experiments is vital for comparing results against known standards. This practice allows researchers to explain the difference between accuracy and precision effectively. For instance, including a blank sample, which ideally has zero concentration of the target analyte, establishes a baseline reference that enhances the reliability of assay results. Studies have shown that calibration errors can lead to significant biases, with potential economic impacts ranging from 8 to 31 USD per patient due to an analytical bias of 0.1 mg/dL.
Standardization of Protocols: Creating and following standardized protocols for data collection reduces variability and improves consistency in assessment. This standardization is essential for enhancing accuracy, as it can help explain the difference between accuracy and precision across various studies and environments.
Training and Competency Assessment: Ensuring that all personnel involved in data collection are adequately trained and regularly assessed for competency can significantly reduce human error. This training is crucial for improving both correctness and exactness in assessments, as experienced staff are less likely to introduce variability.
Statistical Analysis: Employing robust statistical methods to analyze data is fundamental in identifying outliers and assessing the reliability of measurements. Statistical power analyses, for instance, can assist in identifying the smallest sample size required to observe meaningful effects, thus aiding enhancements in both correctness and exactness. It is noteworthy that many studies fail to achieve adequate power, which can compromise the validity of their findings.
By implementing these techniques, researchers can significantly enhance the quality of their clinical studies, leading to more reliable and impactful results.
In clinical research, it is crucial to explain the difference between accuracy and precision, as they are essential across various domains.
Diagnostic Testing: In clinical trials assessing new diagnostic tests, precision is paramount. A test that consistently identifies individuals with a disease (true positives) while correctly recognizing healthy people (true negatives) demonstrates high accuracy. Equally crucial is accuracy itself; if the test produces consistent outcomes across several trials, it signifies high accuracy, which is necessary for trustworthy diagnosis and treatment of individuals.
Drug Efficacy Research: Precise evaluations of individual outcomes, such as blood pressure or cholesterol levels, are essential in studies examining the effectiveness of new medications. Consistent results across varied participant groups and trials indicate precision, thereby strengthening the dependability of the findings. For instance, a diabetes study involving 300 patients highlighted the importance of accurate monitoring, revealing that centralized oversight could uncover discrepancies in vital sign measurements, thereby enhancing the study's integrity.
Clinical Trials for Medical Devices: When evaluating new medical devices, precision ensures that physiological parameters are measured correctly. For example, a glucose monitor that reliably delivers readings near actual blood glucose levels illustrates precision. If it also provides comparable readings across different individuals and situations, it demonstrates accuracy, which is essential for ensuring safety and making informed treatment choices. The interplay between accuracy and precision is vital in these contexts, as explaining the difference between accuracy and precision can prevent inaccuracies that lead to erroneous conclusions and jeopardize patient outcomes.

Understanding the distinction between accuracy and precision in clinical research is vital for ensuring the reliability and validity of study outcomes. Precision relates to the consistency of measurements, while accuracy reflects how closely those measurements align with the true value. Both elements are essential for making informed clinical decisions that impact patient safety and treatment effectiveness.
High precision indicates reliable and repeatable results, whereas high accuracy ensures those results are close to the true values. Key strategies for improving both aspects include:
Each of these techniques contributes to reducing variability and enhancing the overall quality of clinical research.
In conclusion, prioritizing accuracy and precision is not merely a regulatory obligation; it is a fundamental ethical responsibility in clinical research. By adopting rigorous methodologies and continuously striving for improvement, researchers can significantly enhance the integrity of their studies. This commitment ultimately leads to better patient outcomes and advances medical knowledge, reinforcing the critical role that accuracy and precision play in the field of clinical research.
What is precision in clinical research?
Precision in clinical research is defined as the extent to which a recorded value aligns with the genuine or accepted value, indicating the correctness of a calculation. For example, a blood pressure monitor that consistently displays 120/80 mmHg when the true value is also 120/80 mmHg illustrates precision.
How is accuracy defined in clinical research?
Accuracy refers to the consistency of repeated measurements, reflecting how closely these measurements cluster together, regardless of their proximity to the true value. For instance, if a blood pressure monitor gives readings of 118/78 mmHg, 119/79 mmHg, and 121/81 mmHg, it demonstrates consistency but may be inaccurate if the actual value is 120/80 mmHg.
Why is it important to differentiate between accuracy and precision in clinical studies?
Differentiating between accuracy and precision is essential for interpreting information and findings in clinical studies effectively. High levels of both are crucial for obtaining dependable results and making informed clinical decisions.
What can enhance accuracy and reliability in clinical research?
Implementing standardized protocols and regular calibration can significantly enhance both accuracy and reliability in clinical research, ultimately benefiting patient safety and advancing medical knowledge.
What role does bias play in clinical research?
Addressing bias is vital in clinical research as it can profoundly impact the validity of results, potentially leading to erroneous conclusions.