


The interplay between the Department of Health and Human Services (HHS) and the Food and Drug Administration (FDA) significantly shapes the landscape of clinical research in the United States. This dynamic influences everything from ethical standards to approval timelines, presenting both opportunities and challenges for researchers. Each agency's distinct regulatory frameworks require careful navigation of complex requirements.
As the number of clinical trials continues to surge, stakeholders must effectively balance the rigorous ethical considerations mandated by HHS with the expedited processes favored by the FDA. How can researchers streamline their studies while ensuring compliance and participant safety? Understanding these key differences is essential for success in this evolving field.
The roles of HHS vs FDA are crucial in the U.S. regulatory framework, with each agency having distinct responsibilities that significantly impact medical research. HHS is tasked with overseeing public health and welfare, tackling a wide array of health-related issues, including the protection of human subjects through regulations like the Common Rule. This regulation sets forth ethical standards for research involving human participants, ensuring that their rights and welfare are prioritized.
On the other hand, the FDA focuses on ensuring the safety and efficacy of drugs, biologics, and medical devices. The challenges in compliance for researchers arise from the regulatory structure maintained by the FDA under HHS, highlighting the complexities of HHS vs FDA. For instance, the comparison of HHS vs FDA reveals that the FDA's stringent criteria for drug approval often necessitate extensive medical trials, while HHS regulations may place greater emphasis on ethical considerations and participant protections.
These distinctions can lead to complexities in compliance, especially when navigating the HHS vs FDA overlapping jurisdictions. A research trial, for example, may need to comply with both HHS's Common Rule and the FDA's regulations, illustrating the challenges in the HHS vs FDA scenario. The dual oversight of HHS vs FDA complicates the approval process and increases the burden on researchers.
Statistics illustrate the impact of these regulatory frameworks on medical studies. As of November 2025, ClinicalTrials.gov lists over 557,292 studies, showcasing the growing landscape of medical investigations that must adhere to both HHS and FDA regulations. Understanding these frameworks, particularly the differences in HHS vs FDA regulations, is essential for stakeholders in medical research, as they must adeptly navigate the complexities of compliance and oversight to ensure successful study execution. Comprehensive clinical trial management services-such as feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting-are vital in addressing these challenges. Experts like Katherine Ruiz, who specializes in regulatory affairs for medical devices and in vitro diagnostics in Colombia, and Ana Criado, a director of regulatory affairs with extensive expertise in biomedical engineering and health economics, underscore the necessity of having skilled professionals to guide researchers through the evolving regulatory landscape. As the FDA emphasizes adherence to the FD&C Act, it is imperative for researchers to stay informed about the changing regulatory environment.
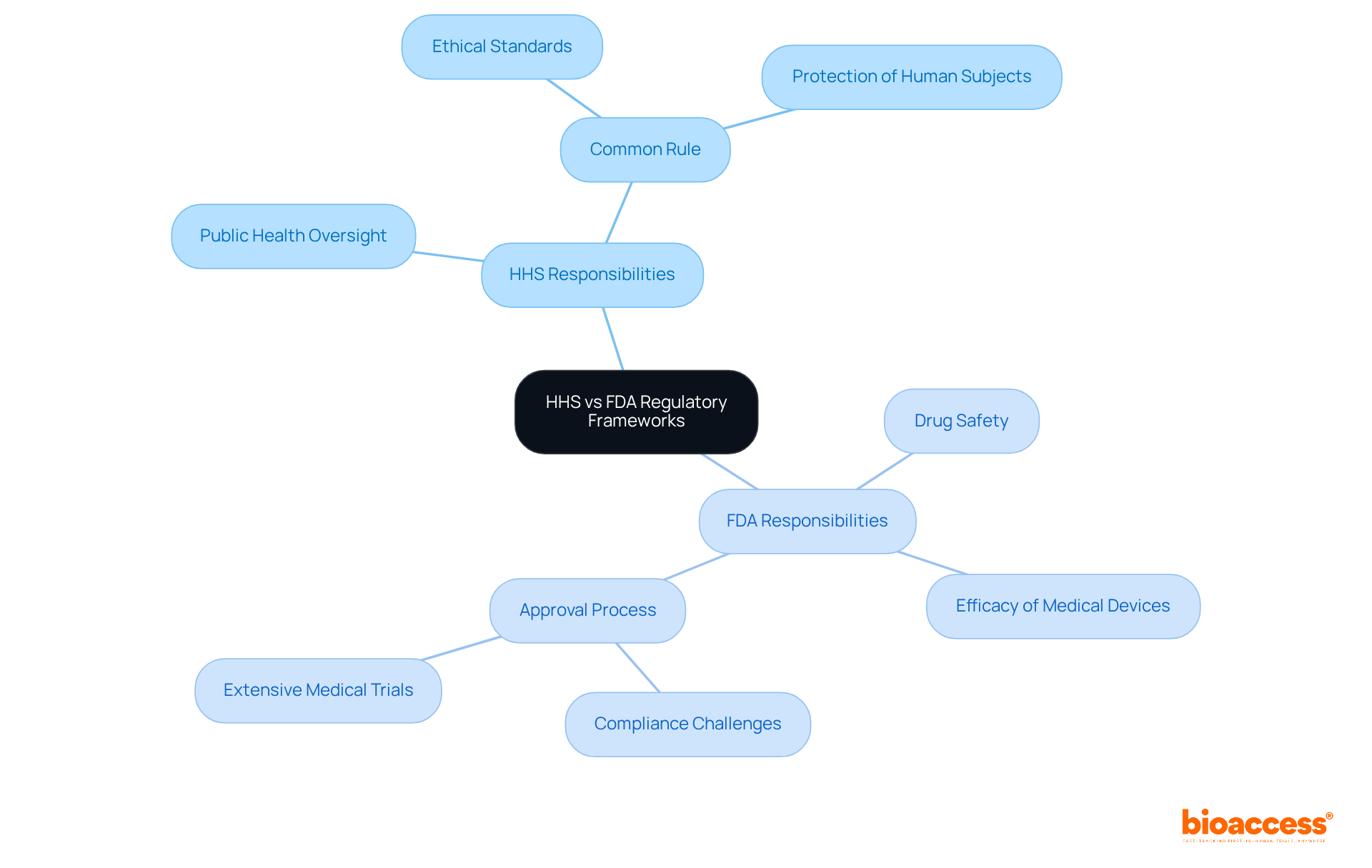
The differences in regulatory frameworks between HHS vs FDA are significant, especially in their approaches to protecting human subjects in clinical research. HHS regulations, as outlined in the Common Rule, mandate that institutions provide assurances and certifications to uphold the ethical treatment of study participants. This framework fosters consistency across federal departments, ensuring adherence to human subject protections in HHS-supported studies. In contrast, FDA regulations emphasize compliance from both sponsors and research investigators, often imposing stricter oversight for studies involving FDA-regulated products. For example, the FDA's expedited review procedures enable certain minimal risk studies to undergo quicker evaluations, enhancing efficiency while ensuring compliance.
This divergence in regulatory focus presents complexities for researchers, particularly in the context of HHS vs FDA jurisdictions, necessitating careful navigation to meet both sets of requirements. Understanding these differences is crucial for effective healthcare management, as it allows researchers to align their studies with the appropriate regulatory standards. By grasping the nuances of HHS and FDA regulations, clinical researchers can better navigate the regulatory landscape, ultimately leading to more efficient and compliant research outcomes.
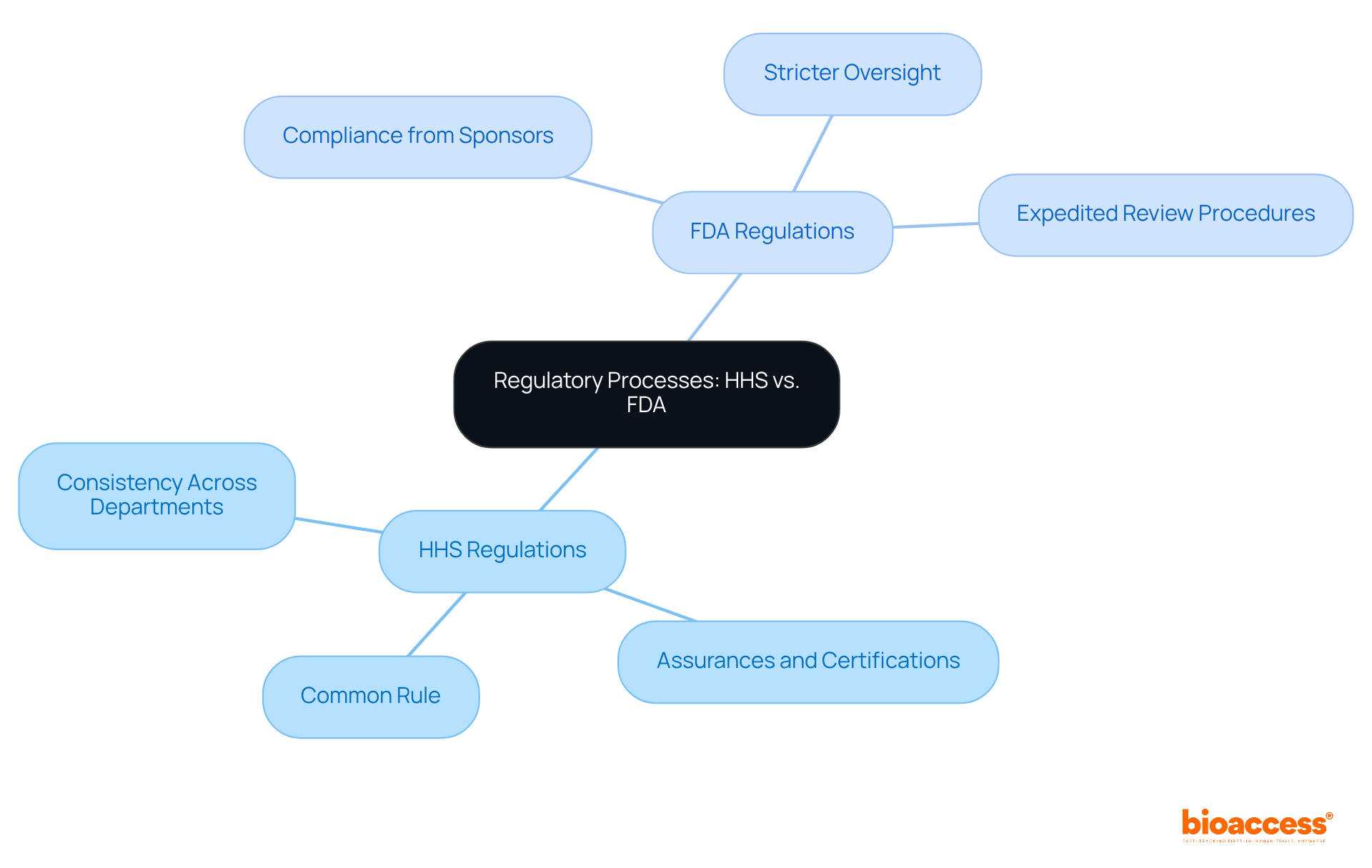
The impact of HHS vs FDA guidelines on medical investigation practices is significant. HHS-funded studies often encounter prolonged approval times due to the need for assurances, which can delay the start of trials. In contrast, the FDA's streamlined processes generally facilitate quicker initiation of trials involving investigational products. For instance, FDA trials can achieve approval times as brief as 4 to 6 weeks, while bioaccess® enables treatment-naive cardiology or neurology cohorts to enroll 50% faster than Western sites, achieving patient enrollment in just 6-8 weeks. This efficiency translates into substantial savings of $25K per patient, thanks to bioaccess®'s FDA-ready data that eliminates rework and delays.
Moreover, the differing definitions of 'research' and 'clinical investigation' in the debate of HHS vs FDA can complicate study design. Researchers must navigate the regulatory landscapes of HHS vs FDA with care to optimize their study designs and ensure compliance, which is essential for expediting the introduction of new therapies to the market. Experts have observed that the expedited pathways established by the FDA, particularly for therapies addressing unmet medical needs, can significantly enhance the speed of drug development. However, this acceleration raises concerns regarding the adequacy of evidence supporting safety and efficacy, especially when surrogate measures are utilized.
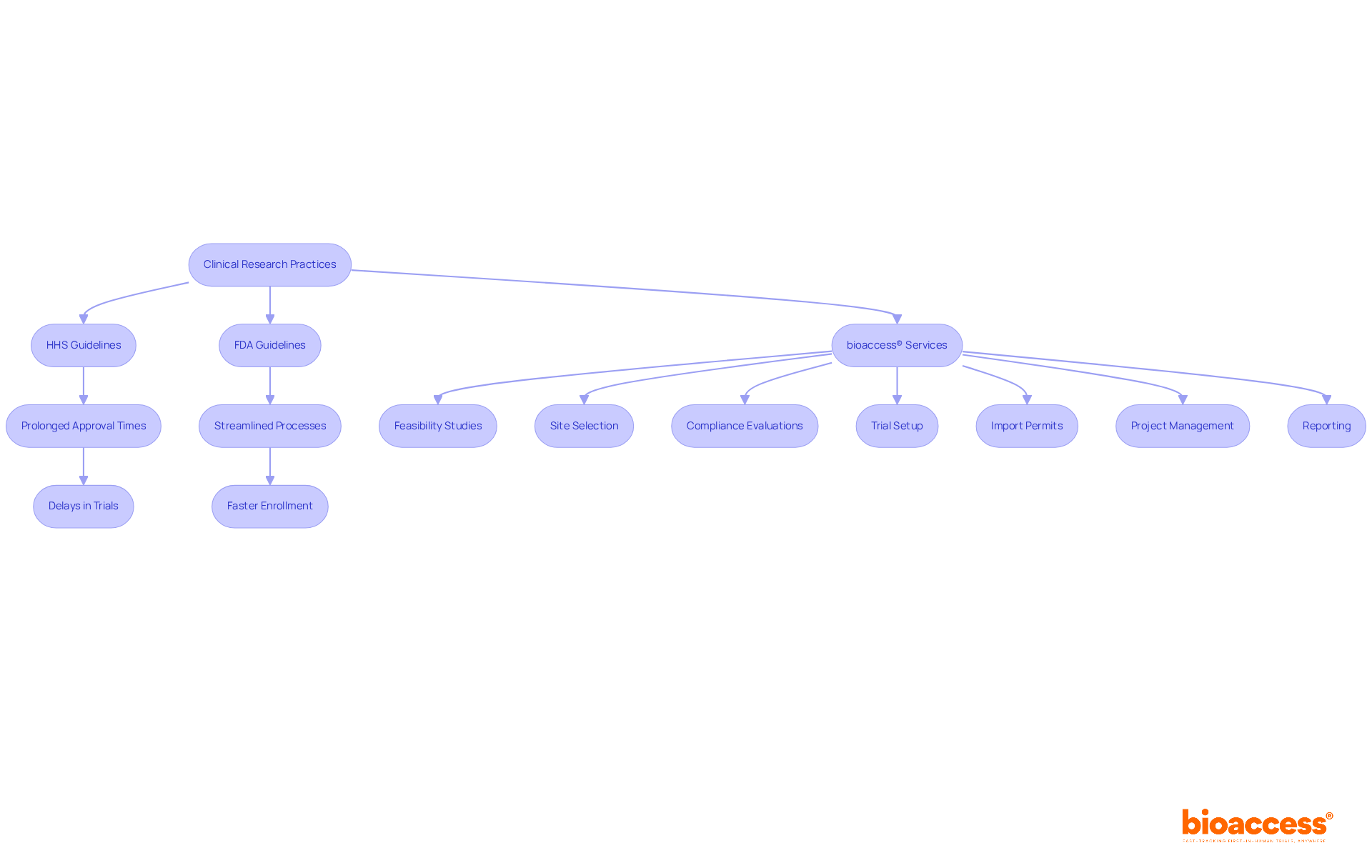
As the landscape of medical studies evolves, understanding these regulatory nuances becomes crucial for investigators aiming to deliver groundbreaking therapies to patients effectively. The ongoing dialogue among stakeholders underscores the necessity for a balanced approach that prioritizes both rapid access to new treatments and rigorous evaluation of their benefits. Furthermore, bioaccess® offers extensive trial management services, including:
These services further streamline the investigation process.
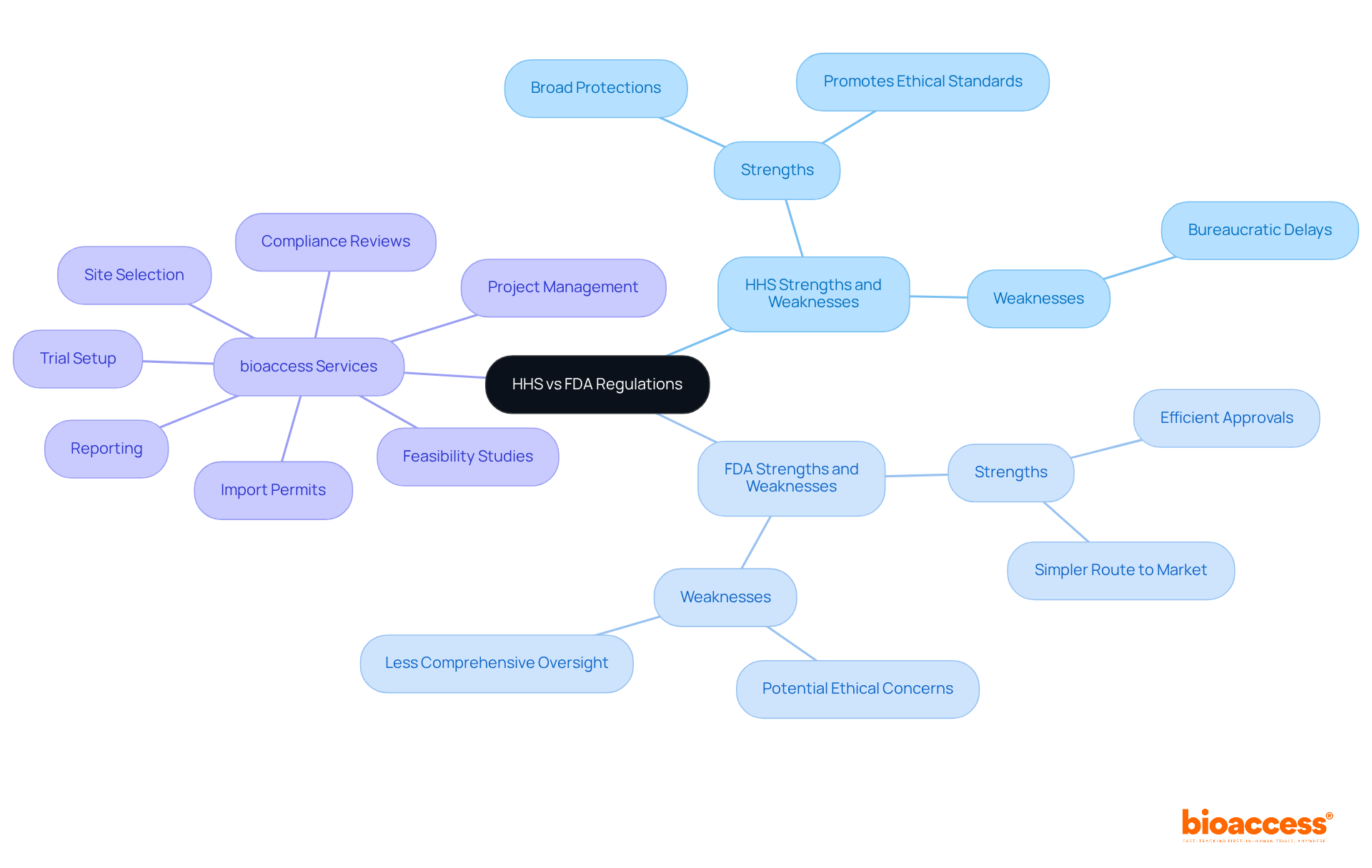
When comparing the strengths and weaknesses of HHS vs FDA, several factors come into play. HHS regulations, particularly the Common Rule, provide broad protections for human subjects across various types of research, promoting ethical standards. However, this can lead to bureaucratic delays that hinder progress. In contrast, FDA regulations are often seen as more efficient for trials involving drugs and devices, allowing for quicker approvals and a simpler route to market. Yet, this efficiency may come at the expense of comprehensive oversight, raising potential ethical concerns.
In this context, bioaccess offers comprehensive clinical trial management services that adeptly navigate these regulatory landscapes. Our capabilities encompass:
For instance, our feasibility studies help identify the most suitable study locations, while our compliance reviews ensure that all study documents meet national requirements, thereby minimizing potential delays. With experts like Katherine Ruiz, who specializes in regulatory affairs for medical devices and in vitro diagnostics in Colombia, we equip our clients to tackle the complexities of both HHS and FDA regulations effectively. Ultimately, the choice between HHS vs FDA regulations hinges on the specific context of the research being conducted, and bioaccess is prepared to support that decision-making process.
Navigating the regulatory landscapes of HHS and FDA is not just essential; it’s a cornerstone of successful clinical research. Their distinct yet overlapping roles in safeguarding public health and ensuring the efficacy of medical products demand a thorough understanding from researchers. This understanding directly influences the ethical treatment of human subjects and the approval processes for investigational products.
Key insights into HHS and FDA regulations reveal the complexities that researchers face. HHS emphasizes ethical standards and participant protections through the Common Rule, while the FDA prioritizes the safety and efficacy of drugs and devices, often resulting in faster trial approvals. This interplay between regulatory bodies creates challenges that necessitate careful compliance. The increasing number of studies listed on ClinicalTrials.gov underscores the demand for adept navigation of these frameworks.
Ultimately, the importance of grasping HHS and FDA regulations cannot be overstated. As the landscape of medical research evolves, researchers must prioritize compliance and ethical considerations to facilitate the introduction of groundbreaking therapies. By leveraging comprehensive clinical trial management services and staying informed about regulatory changes, stakeholders can contribute to a more efficient and responsible research environment. This ensures that innovation continues to thrive while safeguarding the rights and welfare of participants.
What are the primary roles of HHS and FDA in the U.S. regulatory framework?
HHS oversees public health and welfare, addressing various health-related issues, including the protection of human subjects through regulations like the Common Rule. The FDA focuses on ensuring the safety and efficacy of drugs, biologics, and medical devices.
What is the Common Rule?
The Common Rule is a regulation that sets forth ethical standards for research involving human participants, ensuring that their rights and welfare are prioritized.
How do HHS and FDA regulations differ?
The FDA's stringent criteria for drug approval often require extensive medical trials, while HHS regulations emphasize ethical considerations and participant protections.
What challenges do researchers face due to the overlap between HHS and FDA regulations?
Researchers must navigate the complexities of compliance with both HHS's Common Rule and the FDA's regulations, which can complicate the approval process and increase the burden on them.
How many studies are listed on ClinicalTrials.gov, and what does this indicate?
As of November 2025, ClinicalTrials.gov lists over 557,292 studies, indicating a growing landscape of medical investigations that must adhere to both HHS and FDA regulations.
Why is it important for stakeholders in medical research to understand the HHS and FDA frameworks?
Understanding these frameworks is essential for stakeholders as they must navigate the complexities of compliance and oversight to ensure successful execution of studies.
What services are crucial for addressing the challenges posed by HHS and FDA regulations?
Comprehensive clinical trial management services, including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting, are vital for addressing these challenges.
Who are some experts that can assist researchers with regulatory affairs?
Experts like Katherine Ruiz, who specializes in regulatory affairs for medical devices and in vitro diagnostics, and Ana Criado, a director of regulatory affairs with expertise in biomedical engineering and health economics, can guide researchers through the regulatory landscape.
What legislation does the FDA emphasize for researchers to adhere to?
The FDA emphasizes adherence to the Federal Food, Drug, and Cosmetic (FD&C) Act, highlighting the importance for researchers to stay informed about the changing regulatory environment.