


In the intricate landscape of medical device development, Clinical Research Organizations (CROs) emerge as pivotal players, ensuring that clinical trials are conducted efficiently and in compliance with stringent regulatory standards. These specialized entities not only facilitate the management of trials but also enhance data integrity and patient safety, crucial factors that can determine the success of a new medical product. By exploring the multifaceted roles of CROs, the essential steps for conducting clinical research, and the ethical considerations involved, stakeholders can better navigate the complexities of clinical trials.
This article delves into the essential components of clinical research in the medical device sector, offering insights into best practices that can lead to successful outcomes and innovation in healthcare.
Clinical Research Organizations (CROs) are specialized entities that provide crucial support to the pharmaceutical, biotechnology, and medical device industries throughout the development of new products. They play a crucial role in overseeing clinical studies by ensuring adherence to legal standards and facilitating effective data gathering and analysis.
Key roles of CROs involve:
For instance, bioaccess™ offers tailored solutions to navigate the complex regulatory landscape and address challenges such as competition from established players and difficulties in subject recruitment. Their partnership with the Caribbean Health Group seeks to establish Barranquilla as a leading location for medical research in Latin America, supported by Colombia's Minister of Health.
Additionally, partnerships like that of GlobalCare Clinical Trials with bioaccess™ have proven successful, achieving over a 50% reduction in recruitment time and an impressive 95% retention rate.
By comprehending how CROs function and their essential roles, medical device startups can greatly improve the effectiveness and success of their studies, making CROs essential collaborators in research.

Define the Research Objective: Clearly outline the purpose of the clinical trial, including specific objectives and hypotheses related to the medical device. This step establishes the direction for the entire research.
Develop the Protocol: Create a detailed research protocol that includes the design, methodology, inclusion/exclusion criteria, endpoints, and statistical analysis plan. This document is essential for authorization and guiding the execution of experiments.
Select a CRO: Choose a dependable Clinical Research Organization that focuses on medical device evaluations, such as bioaccess®, which has over 20 years of experience in overseeing clinical research in Latin America. Evaluate their expertise, past performance, and resources to ensure alignment with your project goals.
Regulatory Submission: Submit the research protocol to relevant regulatory bodies (e.g., FDA, EMA, and INVIMA in Colombia) for approval. This process may involve preparing additional documentation such as an Investigational Device Exemption (IDE).
Site Selection and Initiation: Identify and initiate study locations where the research will be conducted. This involves selecting qualified investigators and ensuring sites are adequately prepared to follow the protocol, leveraging bioaccess®'s capabilities in feasibility and site selection.
Patient Recruitment: Implement strategies for recruiting eligible participants. This may include advertising, collaborating with healthcare providers, and utilizing patient registries to facilitate enrollment.
Conduct the Experiment: Execute the clinical study according to the protocol, ensuring compliance with Good Clinical Practice (GCP) guidelines. Monitor the experiment closely to address any issues that arise promptly, supported by bioaccess®'s project management services.
Data Collection and Management: Gather, oversee, and assess data throughout the study. Ensure accurate record-keeping and adherence to data integrity standards.
Final Analysis and Reporting: Upon completion of the trial, perform final data analysis and prepare a comprehensive report detailing the findings, conclusions, and implications for the medical device. This is crucial for post-market clinical follow-up studies (PMCF).
Post-Trial Activities: Conduct follow-up activities, including submission of results to oversight bodies like INVIMA and publication in scientific journals. This is crucial for advancing medical knowledge and ensuring patient safety, as well as showing adherence to standards.
In conclusion, adhering to these steps carefully will not only guarantee the effective management of your research study but also assist in navigating the intricate regulatory environment in Colombia, especially under INVIMA's supervision, ultimately resulting in the successful launch of your medical device into the market.

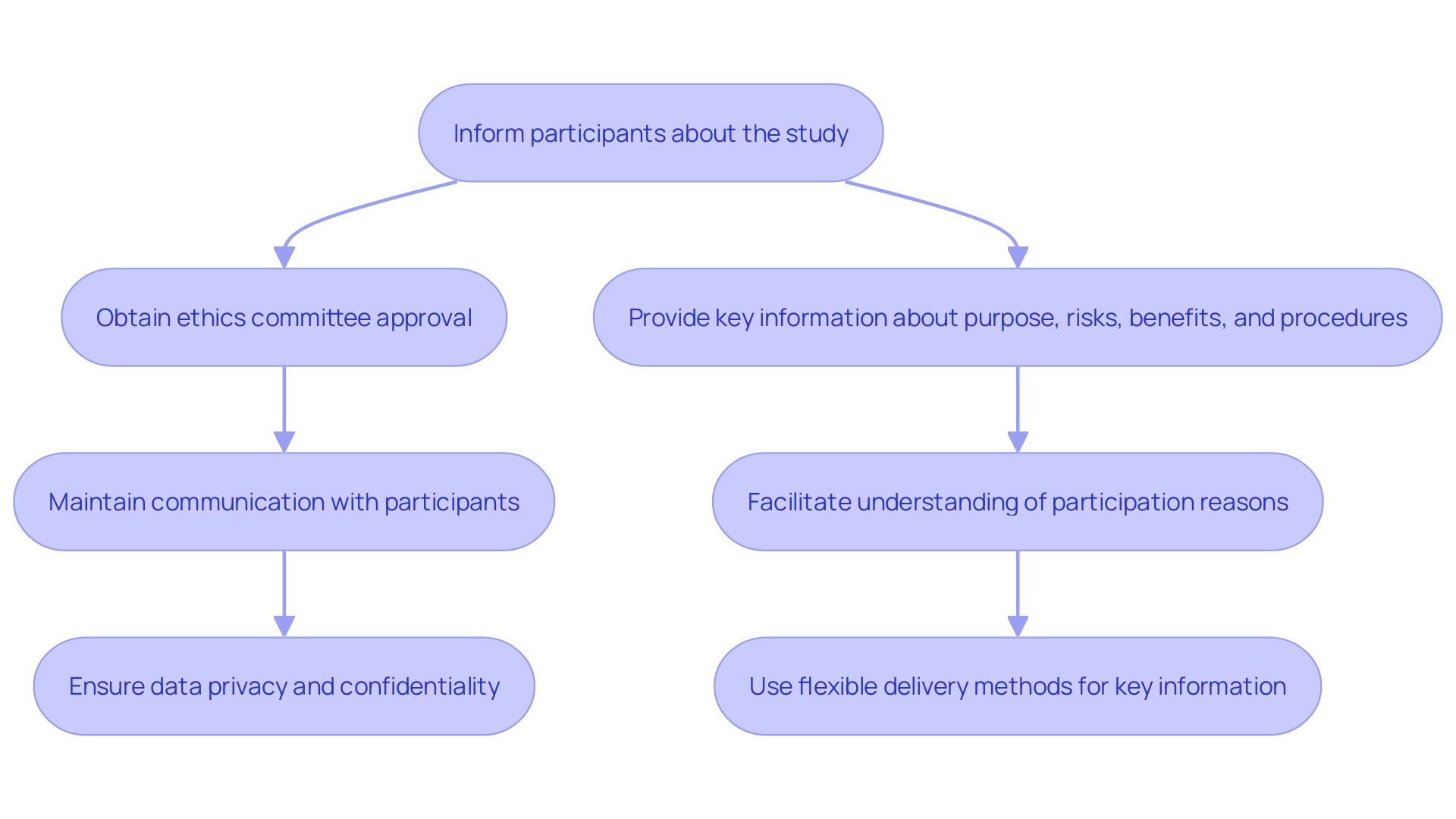
Before commencing any clinical trial, it is essential to establish ethical guidelines and obtain informed consent from participants. This process involves:
Informed Consent Process: Ensure participants are fully informed about the project's purpose, procedures, potential risks, and benefits. Provide them with a clear and understandable consent form that outlines this information.
Ethics Committee Review: Submit the research protocol and informed consent documents to an Institutional Review Board (IRB) or Ethics Committee for evaluation and approval. This helps ensure that the research meets ethical standards and protects participants' rights, in alignment with local regulatory requirements set forth by INVIMA, the Colombian National Food and Drug Surveillance Institute.
Continuous Dialogue: Keep open channels of communication with participants throughout the research. Keep them informed of any changes to the research that may affect their participation and ensure their right to withdraw at any time without penalty.
Data Privacy and Confidentiality: Implement measures to protect participants' personal information and data confidentiality, ensuring compliance with regulations such as HIPAA.
By prioritizing ethical considerations and informed consent, researchers can foster trust and transparency, which are essential for the success of medical studies. Furthermore, through our comprehensive clinical study management services—including feasibility studies, site selection, compliance reviews, setup, and project management—we not only ensure adherence to ethical guidelines but also contribute to the growth of local economies. Clinical studies create jobs, enhance healthcare infrastructure, and promote international collaboration, ultimately leading to improved health outcomes and economic benefits in the communities we serve.
Establish Monitoring Plans: Develop a comprehensive monitoring plan that outlines how the study will be monitored for adherence to the protocol, Good Clinical Practice (GCP), and regulatory requirements. This is crucial when navigating the intricacies of medical studies, particularly in adherence to INVIMA regulations in Colombia, which supervises the marketing and production of health products.
Regular Site Visits: Conduct regular site visits to assess compliance, review data accuracy, and provide support to investigators and site staff. This practice is essential for ensuring efficient project management and monitoring throughout the testing process, aligning with bioaccess®’s commitment to thorough oversight in research studies.
Data Monitoring Committees: Consider forming an independent Data Monitoring Committee (DMC) to oversee study data and ensure participant safety, especially in high-stakes environments such as Early-Feasibility Studies (EFS) and First-In-Human (FIH) experiments. This aligns with bioaccess®'s approach to prioritizing participant safety and data integrity.
Quality Assurance Audits: Implement periodic quality assurance audits to evaluate compliance with the protocol, identify areas for improvement, and ensure data integrity. Such audits are vital for maintaining high standards in clinical trials, particularly those subject to INVIMA's oversight, and reflect the rigorous standards upheld by bioaccess®.
Training and Support: Offer continuous training and assistance to research staff to ensure they are informed of best practices and compliance updates. This improves the overall quality of the research and decreases the chances of mistakes, ensuring that the investigation aligns with INVIMA’s oversight functions and the standards advised by the PAHO/WHO.
By incorporating robust monitoring and quality assurance practices, researchers can enhance the reliability of their trials and contribute to the advancement of medical knowledge. Partnering with firms like bioaccess®, which specialize in accelerated medical device clinical study services in Latin America, ensures that these practices are effectively implemented, leveraging their expertise in navigating regulatory landscapes.

The role of Clinical Research Organizations (CROs) in the development of medical devices is undeniably crucial. They provide essential support throughout the clinical trial process, from site selection to patient recruitment and regulatory compliance. By understanding the key functions of CROs, such as those demonstrated by bioaccess™, stakeholders can significantly enhance the efficiency and success of their trials, ultimately leading to effective and safe medical innovations.
Conducting clinical research involves a systematic approach, beginning with the formulation of a clear research objective and the development of a comprehensive protocol. Following a step-by-step guide ensures that all aspects of the trial are meticulously planned and executed, adhering to both ethical standards and regulatory requirements. The importance of informed consent and ongoing communication with participants cannot be overstated, as they foster trust and transparency essential for the integrity of the research process.
Moreover, the implementation of robust monitoring and quality assurance practices is vital for maintaining high standards in clinical trials. Regular site visits, data monitoring committees, and quality audits contribute to data integrity and participant safety. Collaborating with experienced CROs, such as bioaccess™, can streamline these processes and ensure compliance with local regulations, particularly in regions like Colombia.
In conclusion, the successful management of clinical trials for medical devices hinges on the collaboration between researchers and CROs. By prioritizing ethical considerations, adhering to regulatory frameworks, and implementing rigorous monitoring practices, stakeholders can navigate the complexities of clinical research effectively. This strategic partnership not only enhances the development of innovative medical devices but also promotes overall advancements in healthcare, ultimately benefiting patients and communities alike.
What are Clinical Research Organizations (CROs)?
Clinical Research Organizations (CROs) are specialized entities that support the pharmaceutical, biotechnology, and medical device industries in the development of new products, overseeing clinical studies, ensuring legal compliance, and facilitating data gathering and analysis.
What key roles do CROs perform?
Key roles of CROs include site feasibility and selection, patient recruitment, compliance submissions, and continuous monitoring of study progress.
How do CROs assist in patient recruitment?
CROs implement strategies for recruiting eligible participants, which may involve advertising, collaborating with healthcare providers, and utilizing patient registries to facilitate enrollment.
What is the significance of partnerships involving CROs?
Partnerships, such as those between GlobalCare Clinical Trials and bioaccess™, have achieved significant results, including over a 50% reduction in recruitment time and a 95% retention rate, demonstrating the effectiveness of collaboration in clinical research.
What steps should be followed to conduct a clinical trial for a medical device?
The steps include defining the research objective, developing the protocol, selecting a CRO, regulatory submission, site selection and initiation, patient recruitment, conducting the experiment, data collection and management, final analysis and reporting, and post-trial activities.
Why is informed consent important in clinical trials?
Informed consent is crucial as it ensures participants are fully informed about the study's purpose, procedures, risks, and benefits, fostering trust and transparency in the research process.
What ethical guidelines must be established before a clinical trial?
Ethical guidelines include obtaining informed consent, submitting documents for Ethics Committee review, maintaining continuous dialogue with participants, and ensuring data privacy and confidentiality.
What monitoring practices are essential during a clinical trial?
Essential monitoring practices include establishing monitoring plans, conducting regular site visits, forming Data Monitoring Committees, implementing quality assurance audits, and providing continuous training and support to research staff.
How do CROs contribute to the success of medical device startups?
By understanding CRO functions and roles, medical device startups can enhance the effectiveness and success of their studies, making CROs essential collaborators in research.