


Navigating the regulatory landscape of clinical trials presents a significant challenge, especially when registering a Phase I oncology study with the Therapeutic Goods Administration (TGA) in Australia. This process is vital not only for ensuring compliance and safety but also for accelerating research that could lead to groundbreaking treatments. In this article, readers will find a comprehensive, step-by-step guide designed to demystify the registration process, detailing the necessary documentation and submission pathways.
But what happens when unforeseen challenges arise during this intricate procedure? Understanding potential pitfalls and strategies to overcome them is essential for successful registration and, ultimately, the advancement of oncology research. By addressing these complexities, we aim to empower researchers and stakeholders to navigate this landscape with confidence.
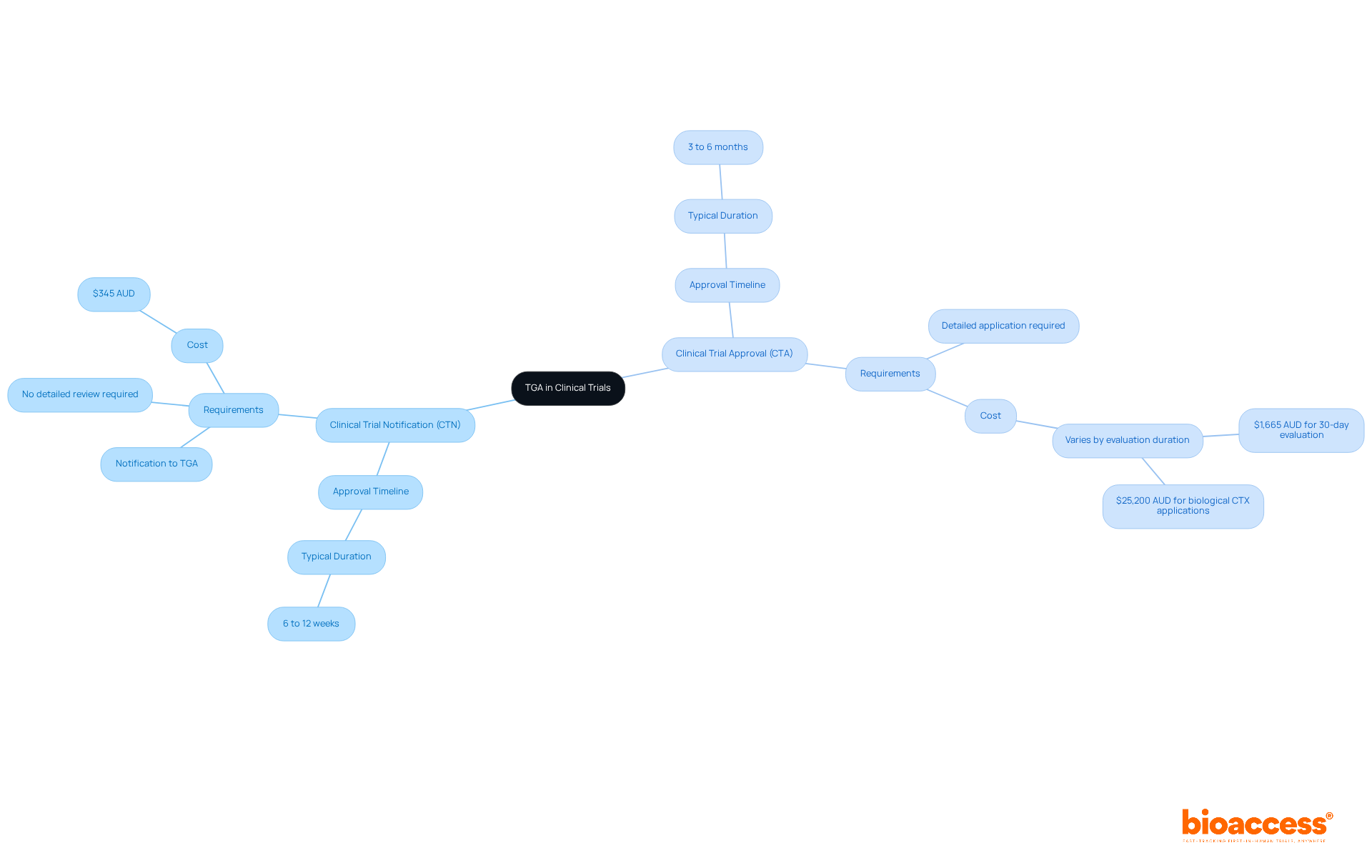
The Therapeutic Goods Administration (TGA) stands as Australia's regulatory authority, dedicated to ensuring the safety, efficacy, and quality of therapeutic goods, including medicines and medical devices. This role is particularly vital in the realm of clinical studies, where the TGA enforces adherence to national regulations and good clinical practice (GCP) standards. Understanding the TGA's operational pathways is essential for navigating the complexities of clinical research.
The TGA primarily operates through two pathways:
This distinction is crucial for organizations aiming to comply with TGA regulations while advancing their research.
Organizations like bioaccess® leverage their expertise in regulatory navigation, patient recruitment, and data management to assist Medtech, Biopharma, and Radiopharma startups in accelerating clinical research outcomes. Recent updates from the TGA underscore ongoing efforts to enhance clinical trial safety and efficacy, highlighting the significance of these regulatory frameworks in fostering successful research outcomes. With bioaccess®'s support, sponsors can transition to the next phase of their research more efficiently, facilitating fundraising and achieving successful exits.
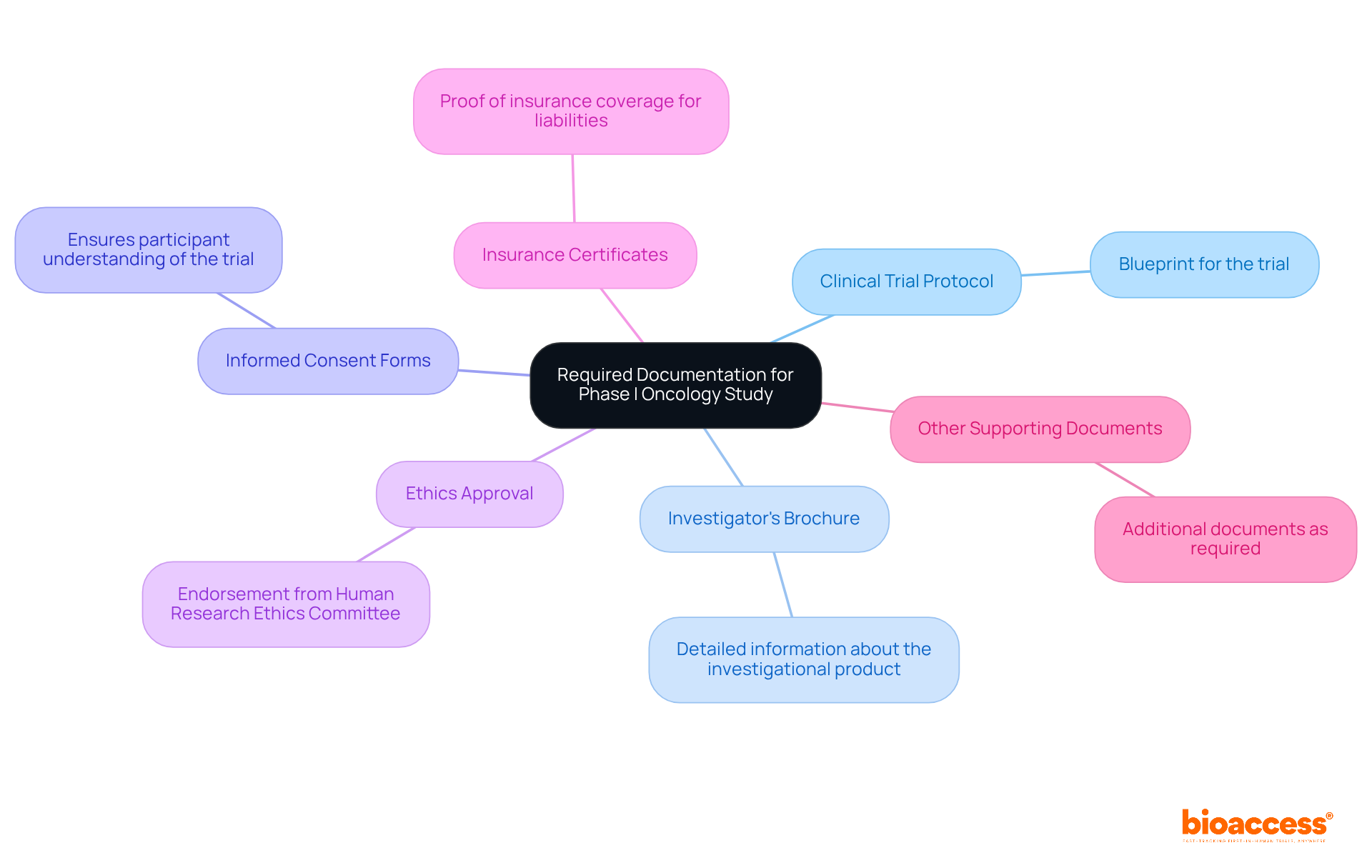
To register a Phase I oncology trial with the TGA, it’s essential to gather several key documents:
In addition to these documents, bioaccess offers extensive clinical trial management services. These include feasibility assessments, site selection, compliance reviews, trial setup, import permits, project management, and reporting. By leveraging bioaccess's expertise, you can understand how to register a phase i oncology study with TGA, ensuring that all documents are prepared in accordance with their guidelines to facilitate a smooth submission process. This collaboration not only streamlines your efforts but also enhances the overall quality of your clinical research.
Once you have gathered all required documentation, the next step is to understand how to register a phase I oncology study with TGA by submitting your application. Here’s how to proceed:
Choose the Appropriate Submission Pathway: Determine whether to utilize the Clinical Trial Notification (CTN) or Clinical Trial Application (CTA) scheme based on your research's specific requirements. The CTN scheme is generally faster and may be suitable for studies with lower risk profiles.
Complete the Application Form: Accurately fill out the relevant application form. For CTN submissions, this usually entails an online form outlining the study, investigational product, and sponsor information.
Submit Supporting Documents: Attach all necessary documents, ensuring they are complete and formatted according to TGA guidelines. This encompasses safety data and ethical approvals, which are essential for a seamless review.
Pay the Submission Fee: Ensure that the appropriate fee is paid at the time of submission. The fee varies depending on the application type, and timely payment is essential to avoid processing delays.
Await Acknowledgment: After submission, you will receive an acknowledgment from the TGA. For CTN submissions, this acknowledgment is typically processed within 10 days, allowing you to track the progress of your application.
Respond to Queries: Be prepared to promptly address any questions or requests for additional information from the TGA. Prompt replies can greatly lessen delays in the approval procedure, ensuring your trial starts as planned.
Successful submissions often hinge on thorough preparation and knowing how to register a phase I oncology study with TGA guidelines. For instance, a recent case study highlighted that sponsors who engaged in pre-submission meetings with the TGA reported a higher success rate in their applications. This proactive approach can clarify expectations and simplify the submission procedure.

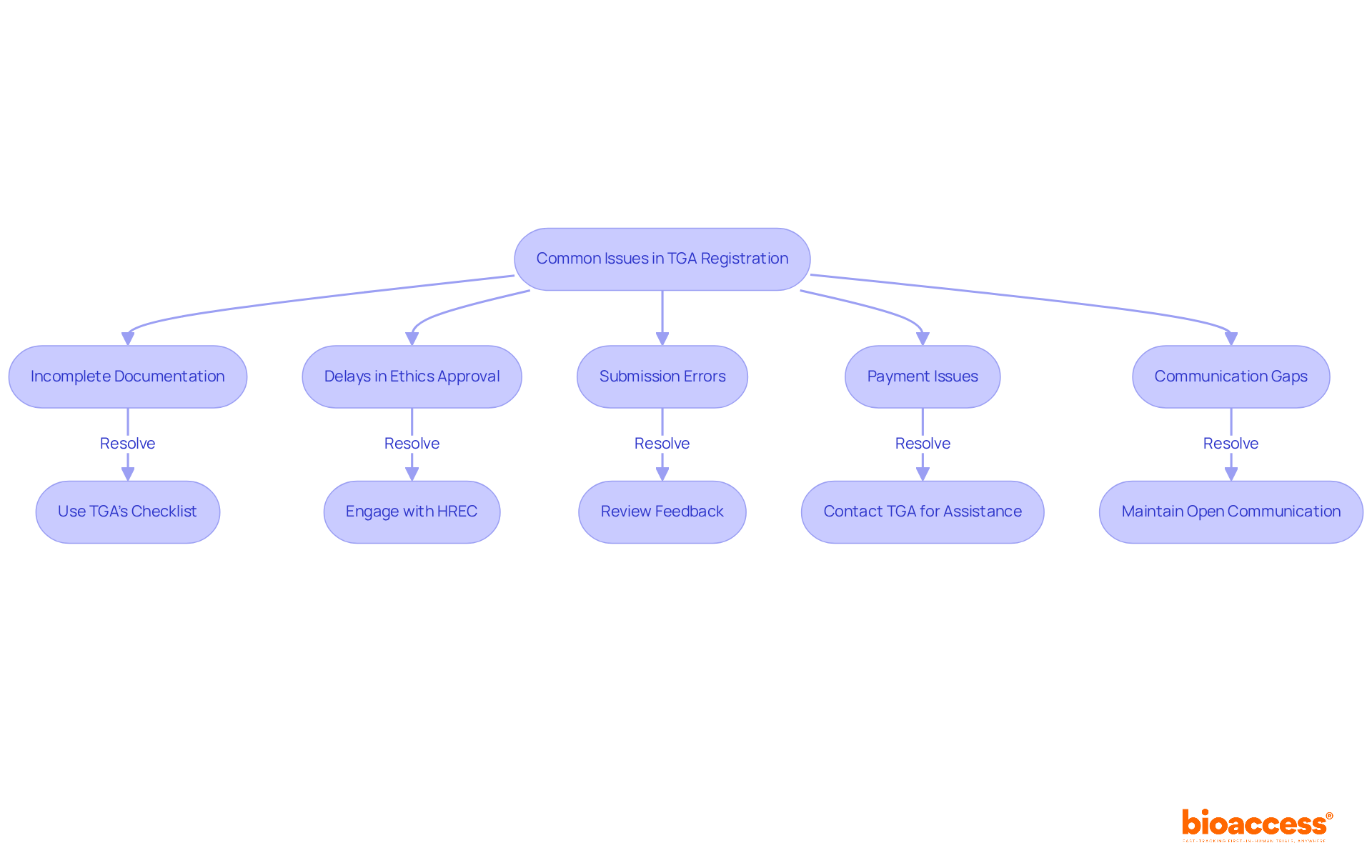
Understanding how to register a phase i oncology study with tga is crucial for clinical research success, yet challenges can arise despite careful preparation. Understanding these key issues and strategies for resolution can significantly enhance your experience:
Incomplete Documentation: Ensure that all necessary documents are included and formatted correctly. Approximately 38% of submissions encounter issues due to incomplete documentation, underscoring the importance of utilizing the TGA's checklist to avoid oversight. As the Therapeutic Goods Administration emphasizes, "Every report is important," highlighting the critical nature of thorough documentation.
Delays in Ethics Approval: If your Human Research Ethics Committee (HREC) approval is postponed, proactively engage with the committee to identify the causes and explore ways to expedite the timeline.
Submission Errors: Upon receiving notifications of errors in your submission, carefully review the feedback and rectify any mistakes before resubmitting to prevent further delays.
Payment Issues: Confirm that the submission fee has been processed correctly. If payment problems arise, promptly contact the TGA for assistance to avoid hindrances in your application.
Communication Gaps: Foster open communication with the TGA. As the TGA advises, "Ensure the nominated contact individual is available throughout the evaluation phase, to respond to any questions the TGA may have." Should you have questions or require clarification, do not hesitate to reach out to their support team, as maintaining dialogue can significantly enhance the registration experience.
By adopting a proactive approach and being prepared to troubleshoot, you can learn how to register a phase i oncology study with tga more efficiently.
Successfully registering a Phase I oncology study with the TGA is crucial for advancing clinical research. A comprehensive understanding of the regulatory landscape and meticulous preparation are essential. By grasping the TGA's role and the specific pathways available for clinical trials, organizations can navigate the complexities of the registration process more effectively. This journey involves not only understanding the distinctions between the CTN and CTA schemes but also emphasizes the importance of thorough documentation and proactive communication.
Key steps for registration include gathering essential documents such as:
While also detailing the submission process itself. Common issues, such as incomplete documentation and delays in ethics approval, can hinder progress. However, strategies to mitigate these challenges exist, and engaging with resources like bioaccess® can further streamline efforts and enhance the likelihood of successful submissions.
The significance of a well-structured approach to TGA registration cannot be overstated. By prioritizing thorough preparation and maintaining open lines of communication with the TGA, sponsors can facilitate smoother trials and contribute to the advancement of oncology research. Embracing these practices will pave the way for successful outcomes, ensuring that vital studies can progress efficiently and effectively within the regulatory framework.
What is the role of the TGA in clinical trials?
The Therapeutic Goods Administration (TGA) is Australia's regulatory authority responsible for ensuring the safety, efficacy, and quality of therapeutic goods, including medicines and medical devices. This role is crucial in clinical studies, where the TGA enforces adherence to national regulations and good clinical practice (GCP) standards.
What are the two main pathways the TGA operates through for clinical trials?
The TGA operates through two main pathways: the Clinical Trial Notification (CTN) scheme, which allows sponsors to notify the TGA of their intent to conduct a trial with an approval timeline of 6 to 12 weeks, and the Clinical Trial Approval (CTA) scheme, which requires a more detailed application and typically takes 3 to 6 months for approval.
How does the CTN scheme differ from the CTA scheme?
The CTN scheme is a streamlined process that requires sponsors to notify the TGA of their trial intent, resulting in quicker approvals (6 to 12 weeks). In contrast, the CTA scheme requires a more comprehensive application, leading to a longer approval timeline of 3 to 6 months due to in-depth evaluation.
Why is understanding the TGA's operational pathways important for organizations?
Understanding the TGA's operational pathways is essential for organizations to comply with TGA regulations while advancing their research, ensuring they follow the correct procedures for clinical trials.
How does bioaccess® assist organizations in clinical research?
Organizations like bioaccess® leverage their expertise in regulatory navigation, patient recruitment, and data management to help Medtech, Biopharma, and Radiopharma startups accelerate clinical research outcomes.
What recent updates from the TGA are mentioned in the article?
Recent updates from the TGA focus on enhancing clinical trial safety and efficacy, underscoring the importance of regulatory frameworks in achieving successful research outcomes.