


ISO 14971 stands as a pivotal standard in medical device risk management, offering a structured framework for identifying hazards, assessing risks, and implementing mitigation strategies throughout a product's lifecycle. Adhering to this standard not only enhances patient safety by proactively addressing potential risks but also facilitates regulatory compliance and elevates manufacturers' credibility within the healthcare sector. This multifaceted approach underscores the critical role that ISO 14971 plays in fostering a safer medical environment.
Understanding the intricacies of medical device safety is more crucial than ever, particularly as the healthcare landscape transforms with emerging technologies and evolving regulatory demands. ISO 14971 stands out as a pivotal standard, providing a comprehensive framework for risk management in the design and development of medical devices. By delving into the principles of ISO 14971, manufacturers can enhance patient safety while streamlining their compliance processes.
However, what challenges do they face in effectively integrating these standards into their operations? Moreover, how can they ensure ongoing adherence in a rapidly changing environment?
The iso definition medical for ISO 14971 is a pivotal global standard that outlines the management process for the design and development of medical equipment. It establishes a structured system for manufacturers to identify potential hazards, assess and evaluate associated risks, and implement effective measures to mitigate those risks. This standard underscores the necessity of a systematic approach to risk management throughout the entire lifecycle of a medical product, in accordance with the iso definition medical, from the initial concept to post-market surveillance. By adhering to the iso definition medical standards, manufacturers not only fulfill regulatory requirements but also demonstrate a robust commitment to the safety and efficacy of their products.
Current adoption rates of ISO standards in medical equipment manufacturing reflect a growing recognition of its importance, with numerous companies integrating its principles into their quality management frameworks. Key components of the iso definition medical standards include risk evaluation, risk management, and the imperative for continuous monitoring and assessment, ensuring that products remain safe and effective throughout their lifecycle.
Real-world applications of the iso definition medical and ISO standards are observable at various stages of medical device development, where manufacturers employ its guidelines to navigate intricate regulatory environments and bolster product safety. In Colombia, experts like Ana Criado, who possesses extensive experience in regulatory affairs, emphasize the significance of ISO standards in aligning local practices with international benchmarks. Recent updates regarding the iso definition medical standards compliance highlight an increasing focus on post-market monitoring and the incorporation of data analytics for real-time risk assessment.
Expert opinions stress that the successful implementation of the iso definition medical guidelines is vital for maintaining high safety standards in medical equipment. Ongoing education and training on compliance practices are crucial for cultivating a culture of safety and ensuring that manufacturers can swiftly adapt to evolving regulatory demands, particularly within Colombia's dynamic healthcare landscape.
The iso definition medical is paramount in underscoring the significance of safety in medical devices. By implementing the risk management processes outlined in the standard, manufacturers can proactively identify potential hazards and take necessary actions to mitigate risks before they culminate in adverse events. This practice not only safeguards patients but also enhances the manufacturer's credibility in the eyes of regulatory bodies and the public.
Furthermore, adherence to the iso definition medical standards is often essential for securing regulatory approvals across various jurisdictions, making it a vital component of the product development process. Ultimately, commitment to this standard fosters a culture of safety and quality within the medical equipment industry.
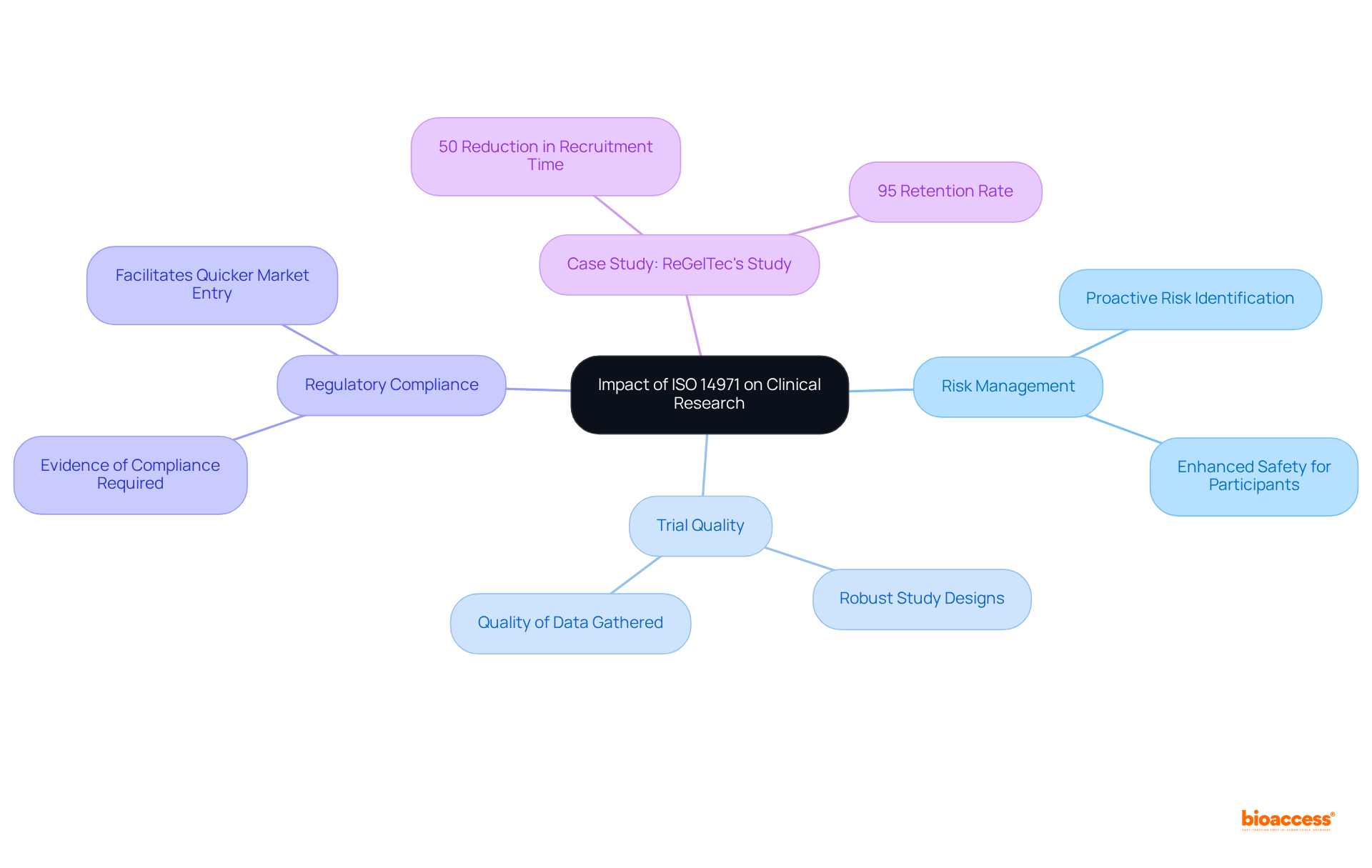
The iso definition medical significantly shapes clinical research and development, guiding the design and execution of clinical trials for medical devices. By incorporating risk management principles into the research process, manufacturers can proactively identify and address potential risks, resulting in more robust study designs. This forward-thinking approach not only enhances the safety of trial participants but also elevates the quality of data gathered during the studies.
For example, ReGelTec's Early Feasibility Study on HYDRAFIL™ for chronic low back pain in Colombia exemplifies how adherence to ISO standards can optimize clinical trials. Through a partnership with bioaccess, the study achieved over a 50% reduction in recruitment time while maintaining an impressive 95% retention rate.
Additionally, regulatory bodies often require evidence of compliance with the iso definition medical standards as part of the clinical trial application submission process. Therefore, aligning with these standards can facilitate quicker market entry for innovative medical products.
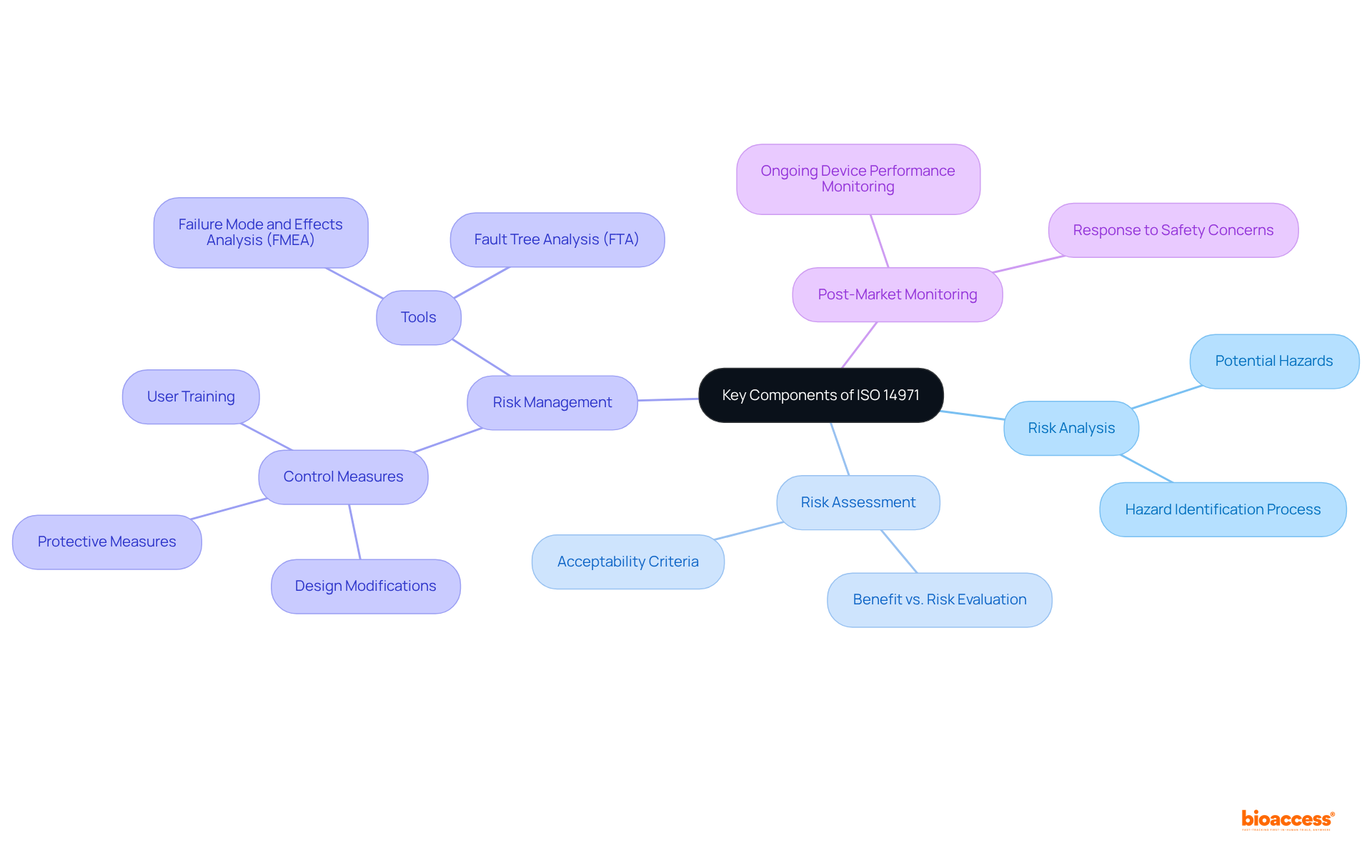
The iso definition medical in ISO 14971 delineates essential elements for the effective management of hazards in medical instruments, which includes the analysis of potential issues, assessment of threats, control measures, and post-market monitoring. Risk analysis is the critical process of identifying potential hazards associated with a medical device, which is vital for ensuring patient safety. For instance, manufacturers can significantly reduce product recall rates—by as much as 75%—by establishing robust management systems that adhere to ISO standards.
Following this, risk assessment evaluates the significance of identified threats against established acceptable criteria. This step is crucial, as it aids in determining whether the benefits of a product outweigh its risks, a necessity underscored in the latest updates of ISO 14971, released in December 2019. The standard has evolved to enhance the focus on post-market surveillance, ensuring that manufacturers continually monitor device performance and respond swiftly to any emerging safety concerns.
Risk management entails the implementation of measures to mitigate identified hazards, which may include design modifications, protective measures, or improved labeling. Tools such as Failure Mode and Effects Analysis (FMEA) are indispensable for prioritizing safety actions based on potential failure modes, ensuring that risks are effectively addressed throughout the product lifecycle.
Collectively, these components constitute a comprehensive risk management framework that is not only essential for the safe development and utilization of medical devices but also aligns with regulatory requirements, ultimately improving patient outcomes and fostering innovation within the healthcare sector.
The significance of ISO 14971 in the realm of medical device safety is paramount. This standard provides a crucial framework for manufacturers, guiding them in the identification, assessment, and management of risks associated with medical devices. By implementing ISO 14971, companies not only comply with regulatory requirements but also prioritize patient safety, fostering a culture of quality throughout the product lifecycle.
Key insights highlighted throughout the article include:
Real-world applications demonstrate how adherence to these standards can lead to enhanced product safety, reduced recall rates, and expedited market entry for innovative devices. The evolving nature of ISO 14971, particularly its focus on post-market surveillance and data analytics, underscores its relevance in maintaining high safety standards.
Ultimately, embracing ISO 14971 transcends a mere regulatory obligation; it represents a commitment to excellence in medical device development. Manufacturers are encouraged to fully integrate these risk management principles into their operations, ensuring that safety and efficacy remain at the forefront of their innovations. By doing so, they not only protect patients but also enhance their credibility and competitiveness in the global market, paving the way for a safer healthcare environment.
What is ISO 14971?
ISO 14971 is a global standard that outlines the risk management process for the design and development of medical devices. It provides a structured system for manufacturers to identify hazards, assess risks, and implement measures to mitigate those risks throughout the product lifecycle.
Why is ISO 14971 important for medical device manufacturers?
ISO 14971 is important because it helps manufacturers fulfill regulatory requirements and demonstrates their commitment to the safety and efficacy of their products. It establishes a systematic approach to risk management from the initial concept to post-market surveillance.
What are the key components of ISO 14971?
Key components of ISO 14971 include risk evaluation, risk management, and the need for continuous monitoring and assessment to ensure that medical devices remain safe and effective throughout their lifecycle.
How is ISO 14971 applied in real-world medical device development?
Manufacturers apply ISO 14971 guidelines at various stages of medical device development to navigate regulatory environments and enhance product safety, ensuring compliance with international standards.
What recent trends are observed regarding ISO 14971 compliance?
Recent trends include an increased focus on post-market monitoring and the use of data analytics for real-time risk assessment, reflecting a growing recognition of the importance of ISO standards in medical equipment manufacturing.
What role do experts play in the implementation of ISO 14971?
Experts emphasize the significance of ISO standards in aligning local practices with international benchmarks and stress that successful implementation is vital for maintaining high safety standards in medical equipment.
Why is ongoing education and training important for manufacturers regarding ISO 14971?
Ongoing education and training are crucial for cultivating a culture of safety and ensuring that manufacturers can adapt to evolving regulatory demands, particularly in dynamic healthcare environments like Colombia.