


This article delineates critical steps for compliance with 21 CFR 820.30, underscoring the necessity of robust design control processes to guarantee the safety and efficacy of medical devices. It asserts that adherence to this regulation not only mitigates regulatory risks—evidenced by the substantial number of Warning Letters issued for specification deficiencies—but also elevates overall product quality through meticulous documentation, cross-functional collaboration, and continuous improvement practices.
Navigating the complexities of medical device regulation can be daunting, particularly with the stringent requirements set forth by 21 CFR 820.30. This regulation stands as a cornerstone of the FDA's Quality System Regulation, outlining critical design control processes that manufacturers must adhere to in order to ensure the safety and efficacy of their products. As the number of Warning Letters issued by the FDA continues to rise, the stakes for compliance have never been higher.
How can manufacturers not only meet these regulatory demands but also leverage them to enhance product quality and market readiness? This article delves into essential steps for mastering 21 CFR 820.30, offering valuable insights that can transform compliance from a burden into a strategic advantage.
The regulation 21 CFR 820.30 is pivotal within the FDA's Quality System Regulation (QSR), necessitating comprehensive management processes for medical devices. This regulation delineates critical procedures that manufacturers must adopt to guarantee the safety and efficacy of their devices. Essential elements encompass:
In the fiscal year 2024, the FDA issued 47 Warning Letters to medical device producers, with deficiencies related to specifications emerging as the most frequently cited issues, accounting for 21 of these letters. This statistic underscores the regulation's vital role in ensuring product safety and highlights the pressing need for manufacturers to refine their processes to evade regulatory scrutiny.
The effective implementation of specifications not only ensures compliance with the requirements of 21 CFR 820.30 but also significantly enhances the overall quality management framework of medical devices. Regulatory specialists emphasize that robust development guidelines are essential for meeting user requirements and intended applications, particularly for Class II and Class III devices. As articulated by the U.S. FDA, "medical device development regulations govern the creation process to ensure that devices fulfill user needs, intended purposes, and specified criteria."
Understanding and adhering to these requirements is paramount for manufacturers aiming to navigate the complexities of regulatory standards effectively. By prioritizing regulatory measures, companies can substantially reduce the likelihood of receiving Warning Letters and improve their product development outcomes. Furthermore, upcoming training sessions, such as the one on Combination Products in February 2025, will offer invaluable resources like the Medical Device Warning Letter Navigator, equipping manufacturers with insights to better tackle compliance challenges.
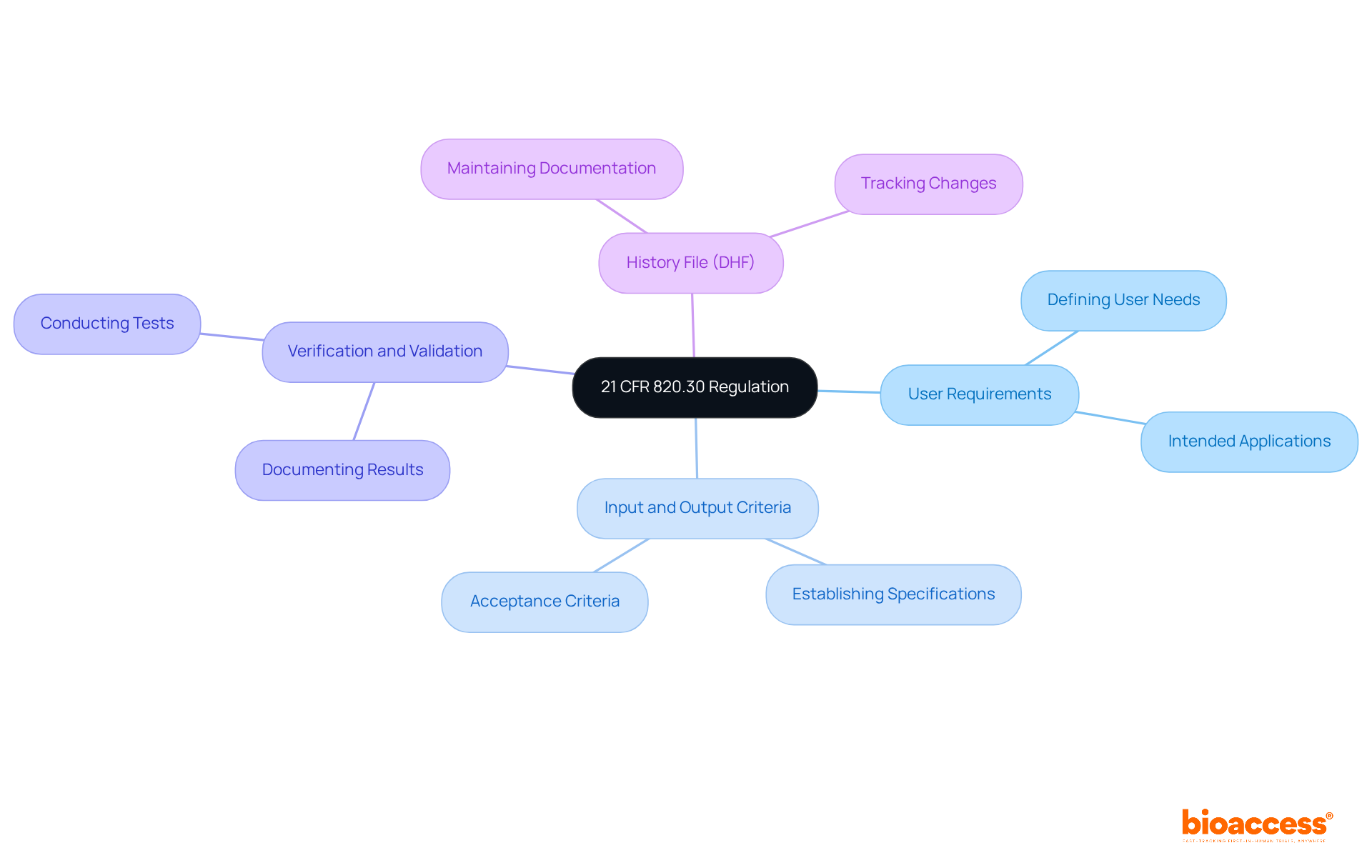
To comply with 21 CFR 820.30, manufacturers must prioritize several essential design control requirements:
Creation and Development Strategy: Formulate a thorough plan that specifies the creation methodology, detailing responsibilities, timelines, and connections among various teams. This organized method is essential for guaranteeing that all elements of the layout are tackled systematically.
Design Input: Clearly define and document user needs and intended uses of the device. This step is crucial, as it establishes the foundation for the development process, ensuring that all requirements are measurable and objective. Significantly, human factors analysis must also be incorporated under input requirements, as required by 21 CFR 820.30.
Output: Ensure that outputs correspond with the established input requirements. Proper documentation of these outputs is necessary, as they serve as the preliminary Device Master Record (DMR), guiding the assembly and production of the medical device.
Validation: Perform comprehensive validation activities to ensure that the outputs correspond to the outlined input requirements. This procedure is essential for demonstrating that the device has been designed correctly and adheres to regulatory standards.
Validation: Confirm the layout to ensure that the device meets user needs and intended uses under actual or simulated conditions. This step is critical for confirming that the device performs as expected in real-world scenarios.
Design History File (DHF): Maintain a thorough DHF that records the complete development process, including choices made and modifications executed throughout the progression. A current DHF is essential for regulatory adherence and successful audits. Neglecting to update the DHF can lead to complications at the Design Transfer stage.
Recent statistics suggest that approximately [insert percentage] of manufacturers struggle to meet input requirements as outlined in 21 CFR 820.30, highlighting the importance of thorough planning and documentation practices. Insights from industry leaders, including Jon Speer, underscore that efficient planning for development not only simplifies compliance but also enhances product quality and safety. By adhering to these requirements, manufacturers can ensure a smoother path to market and mitigate risks associated with regulatory non-compliance.
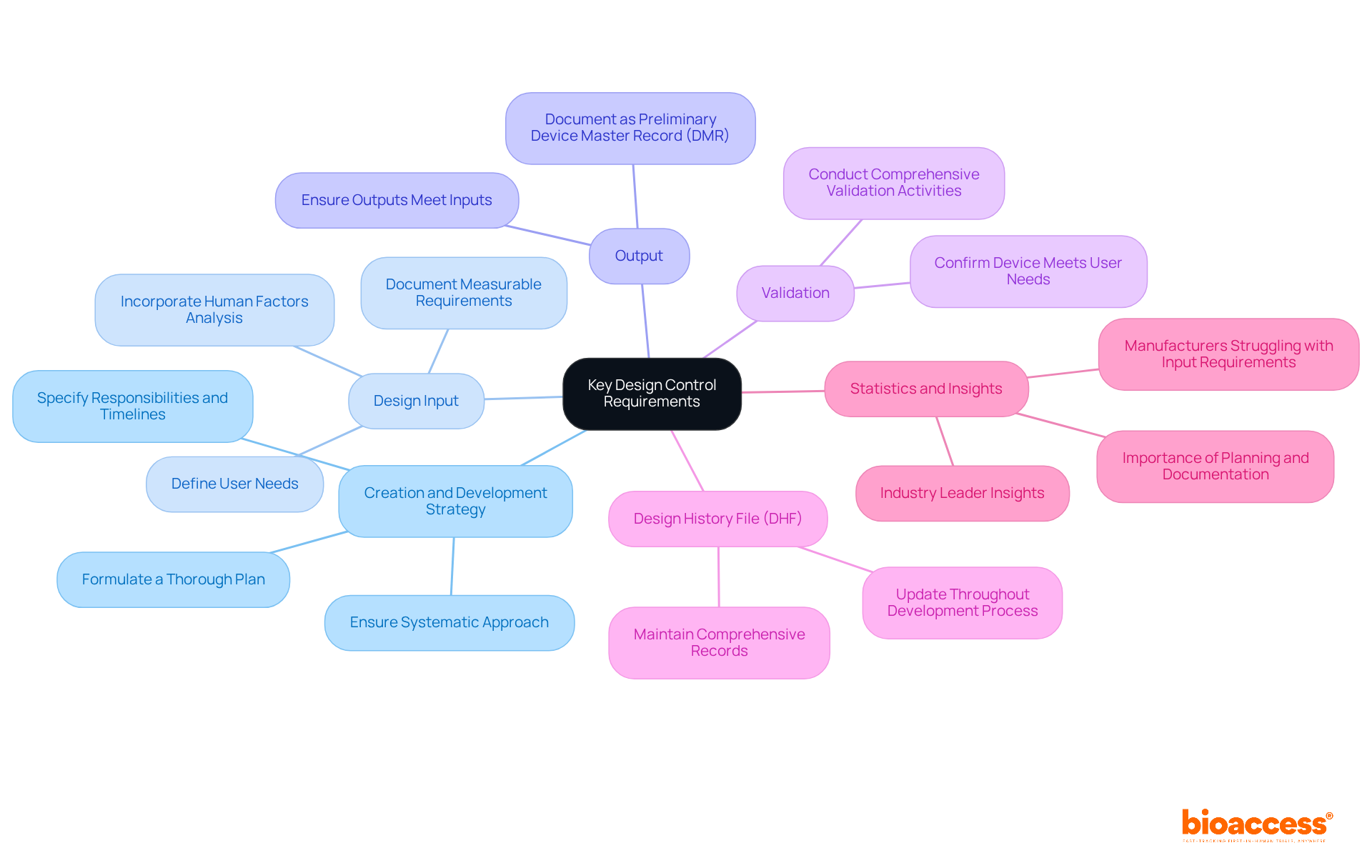
To implement effective design control processes, it is essential to follow these crucial steps:
By following these steps, manufacturers can establish a robust framework for oversight that not only fulfills regulatory requirements but also significantly improves product quality and safety.
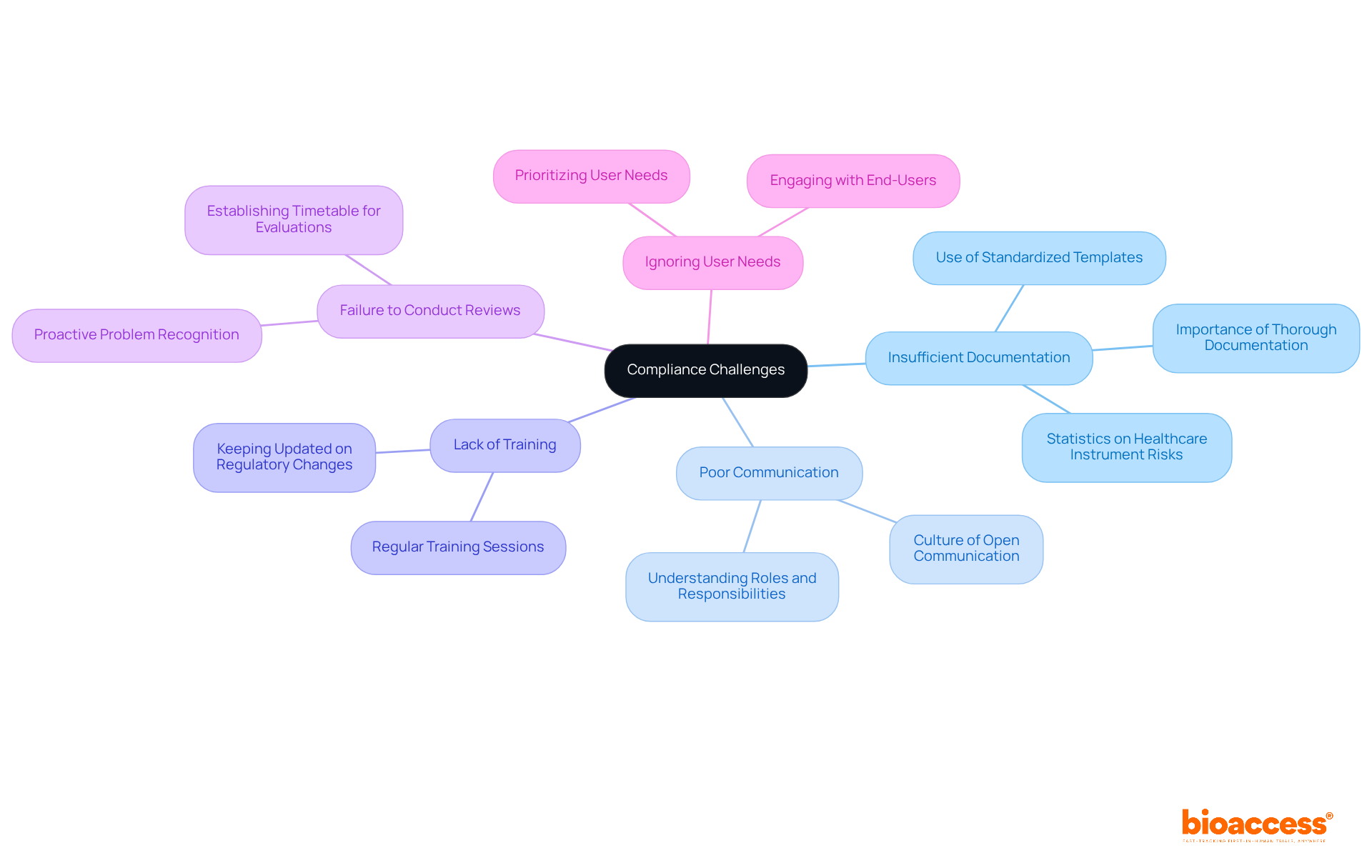
Common compliance challenges in design controls include:
Insufficient Documentation: Thorough documentation of all control activities is essential for demonstrating compliance. Statistics indicate that over 1.7 million injuries and 83,000 deaths in the U.S. are potentially linked to healthcare instruments over a decade, underscoring the critical importance of robust documentation practices. Employing standardized templates and checklists can simplify this task, ensuring that every aspect is documented precisely.
Poor Communication: Establishing a culture of open communication among team members is vital. This ensures that everyone comprehends their roles and responsibilities, as well as the current status of the development process, which can significantly minimize misunderstandings and mistakes.
Lack of Training: Regular training sessions are necessary to keep team members updated on evolving regulatory requirements and best practices in control systems. Ongoing education fosters an informed workforce capable of effectively managing regulatory complexities.
Failure to Conduct Reviews: Establishing a timetable for regular evaluations enables teams to recognize and address potential problems early in the workflow. This proactive strategy can prevent minor issues from escalating into major regulatory failures.
Ignoring User Needs: Prioritizing user needs and intended uses throughout the design process is crucial for ensuring that the final product aligns with market demands. Engaging with end-users can provide valuable insights that enhance product usability and adherence.
Industry specialists emphasize that strong documentation practices not only mitigate regulatory risks but also elevate overall product quality and safety. Paul Koziarz observes, "You must assess adherence not as a cost, but as a way to save money," highlighting the financial implications of neglecting regulations. By proactively addressing these challenges, manufacturers can significantly bolster their compliance efforts, ultimately increasing the likelihood of successful regulatory approval.
Mastering 21 CFR 820.30 is crucial for medical device manufacturers aiming to ensure compliance with FDA regulations while enhancing product quality. This regulation delineates the essential steps for developing safe and effective medical devices, underscoring the significance of comprehensive documentation, cross-functional collaboration, and rigorous validation processes. By grasping and implementing these requirements, manufacturers can substantially mitigate risks linked to regulatory non-compliance and improve their overall quality management systems.
Key points discussed encompass the critical design control requirements, such as:
Furthermore, the article highlights prevalent pitfalls like poor documentation and insufficient training, emphasizing the necessity for proactive measures to cultivate a culture of compliance within organizations.
Ultimately, the journey toward compliance with 21 CFR 820.30 transcends mere regulatory adherence; it embodies a commitment to excellence in product development and user safety. Manufacturers are urged to perceive compliance as an integral component of their operational strategy, thereby enhancing their capability to deliver safe and effective medical devices to market. By prioritizing these vital steps, organizations can navigate the complexities of regulatory standards with confidence and contribute to a safer healthcare environment.
What is 21 CFR 820.30?
21 CFR 820.30 is a regulation within the FDA's Quality System Regulation (QSR) that outlines essential management processes for medical device manufacturers to ensure the safety and efficacy of their products.
What are the key elements of 21 CFR 820.30?
The key elements include defining user requirements, establishing input and output criteria, conducting verification and validation, and maintaining a Design History File (DHF).
Why is 21 CFR 820.30 important for medical device manufacturers?
It is crucial because it helps manufacturers ensure product safety and compliance with regulatory standards, thereby reducing the likelihood of receiving Warning Letters from the FDA.
What issues were most frequently cited in FDA Warning Letters to medical device producers in 2024?
The most frequently cited issues were deficiencies related to specifications, which accounted for 21 out of the 47 Warning Letters issued.
How does compliance with 21 CFR 820.30 enhance product quality?
Effective implementation of specifications ensures compliance with regulatory requirements and significantly improves the overall quality management framework of medical devices.
What is the significance of robust development guidelines in the context of 21 CFR 820.30?
Robust development guidelines are essential for meeting user requirements and intended applications, particularly for Class II and Class III devices, ensuring that they fulfill the necessary criteria.
What resources are available for manufacturers to improve compliance with 21 CFR 820.30?
Upcoming training sessions, such as the one on Combination Products in February 2025, will provide resources like the Medical Device Warning Letter Navigator to help manufacturers tackle compliance challenges effectively.