


In the complex realm of clinical research, aligning research aims with ethical principles is not just a formality; it’s a cornerstone that builds trust and integrity. As researchers face the intricate task of submitting proposals to multiple national ethics boards, the dual submission process stands out as a transformative strategy. This approach not only expedites approvals but also enhances patient access to innovative therapies. Yet, this method presents its own set of challenges - how can researchers adeptly navigate the varied requirements of different boards while upholding compliance and ethical standards? This article explores the crucial steps and best practices for mastering research aims and maneuvering through the dual submission landscape in clinical research, equipping researchers with the essential tools for success.
In medical research, clearly specifying the objectives of a project is essential. This involves defining primary objectives, such as assessing the efficacy of a new treatment or understanding a disease's progression. Ethical considerations must also be integrated into this process, ensuring that the rights and welfare of participants are prioritized. Researchers should refer to established ethical frameworks, such as the Belmont Report, which outlines principles like respect for persons, beneficence, and justice. By aligning research aims with these ethical principles, researchers can promote trust and integrity in their work.
At bioaccess, we provide comprehensive clinical trial management services that support these ethical considerations. Our capabilities encompass feasibility analyses and the selection of research locations and principal investigators (PIs), ensuring that investigations are carried out in suitable environments. We also conduct compliance assessments of research documents to meet country requirements, facilitating ethical trial setup and approval processes through ethics committees and health ministries. Additionally, our expertise in managing import permits and nationalization of investigational devices ensures that all regulatory aspects are addressed, further safeguarding participant welfare. By integrating these services, bioaccess enhances the ethical framework of clinical research, promoting integrity and trust throughout the research lifecycle.
Key Steps:
The alims and national ethics board dual submission procedure is a pivotal strategy in clinical research, allowing researchers to present proposals to multiple national ethics boards simultaneously. This approach significantly accelerates project timelines across different jurisdictions, simplifying the endorsement process and enhancing patient access to innovative therapies. However, researchers must adeptly navigate the distinct requirements of each board, which can vary considerably.
Key Steps:
Current trends indicate a growing acceptance of alims and national ethics board dual submission, especially in regions like the Balkans and Latin America, where regulatory frameworks are evolving to support more efficient trial processes. For instance, bioaccess™ has played a crucial role in navigating these complexities, collaborating with Caribbean Health Group to position Barranquilla as a key site for trials in Latin America. This initiative, endorsed by Colombia's Minister of Health, aims to enhance the research environment, ultimately benefiting diverse patient populations. By leveraging the dual entry method, researchers can expedite endorsements and elevate the overall quality and inclusiveness of medical research.
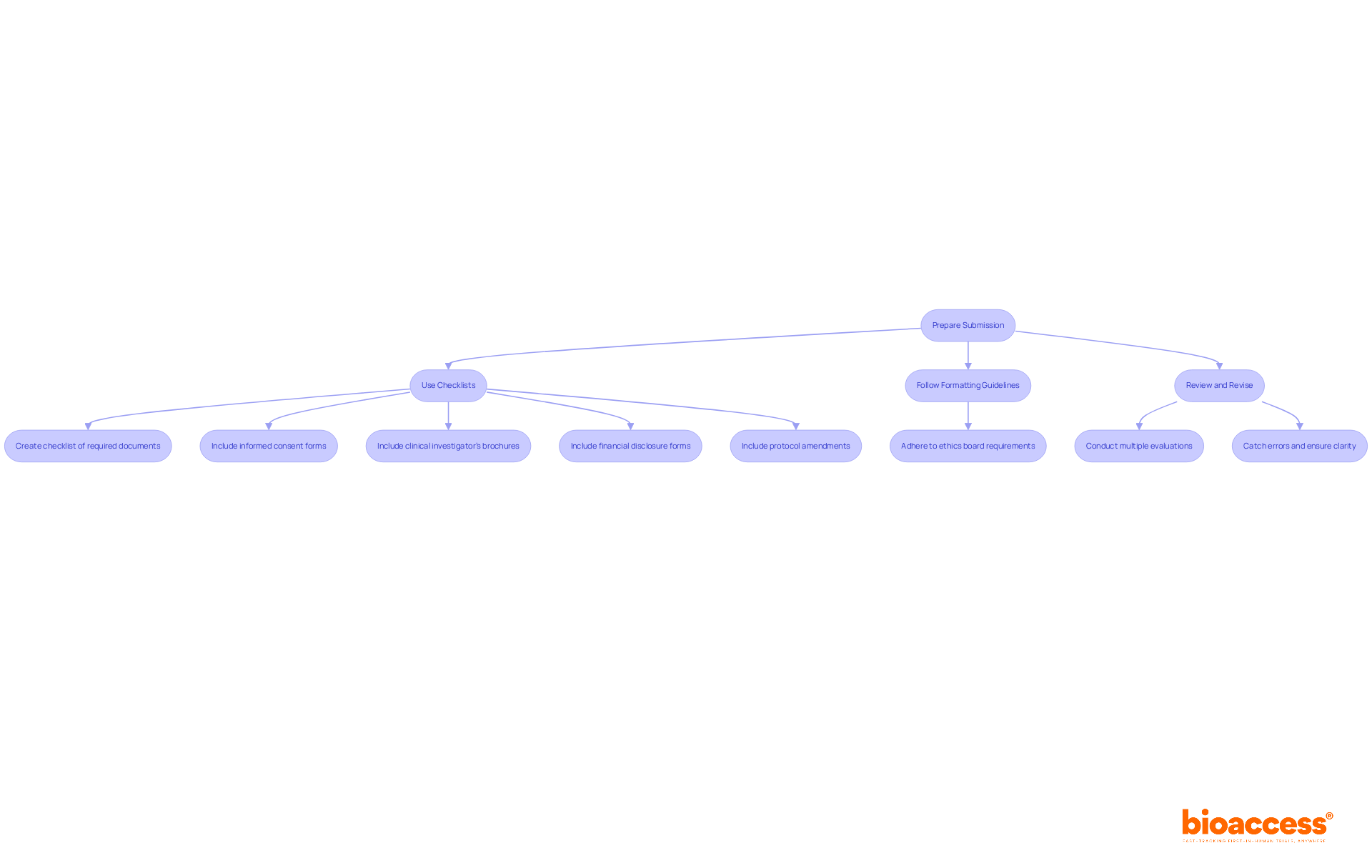
To enhance the likelihood of approval from ethics boards, researchers must adopt effective practices in preparing their proposals. This involves thorough documentation, strict adherence to formatting guidelines, and clear communication with ethics committees.
Key Steps:
The success rates of ethics committees' endorsements are closely tied to the quality of proposals. High-quality documentation not only facilitates quicker approvals but also reflects the research team's commitment to ethical standards. For example, clear and honest participant information sheets significantly enhance participant understanding and decision-making, which is crucial in ethical evaluations.
Expert guidance underscores the importance of comprehensive documentation in ethics proposals. This includes maintaining a regulatory binder that organizes all essential documents, demonstrating compliance with Good Clinical Practice (GCP) and regulatory requirements. As Meghan Hosely emphasizes, a regulatory binder is vital for managing clinical trial documents, ensuring regulatory compliance, and facilitating audits. Furthermore, utilizing electronic regulatory management systems can streamline the documentation process, making it easier to manage and access necessary files.
In summary, a well-prepared application package, characterized by thorough documentation and adherence to guidelines, is essential for successful ethics board endorsements. Researchers should prioritize these practices to enhance their chances of acceptance and uphold ethical integrity in their studies.
While the alims and national ethics board dual submission process can simplify authorizations, it also presents unique challenges, such as differing requirements between ethics boards and potential delays in communication. Researchers must be proactive in addressing these issues to maintain compliance and ensure timely approvals. To support this process, bioaccess offers comprehensive clinical trial management services, including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting. These services assist researchers in effectively navigating the complexities of alims and national ethics board dual submission.
Key Steps:
For instance, in a recent project, bioaccess successfully managed the alims and national ethics board dual submission process for a clinical trial by leveraging its expertise in compliance reviews and project management. This proactive approach minimized delays and ensured that all ethical requirements were met efficiently. By implementing these strategies, researchers can enhance their chances of achieving timely approvals while maintaining the highest ethical standards.
Defining research aims and integrating ethical considerations are essential in clinical research. The dual submission process to national ethics boards not only streamlines approvals but also enhances the integrity and inclusiveness of medical research. By adhering to established ethical frameworks and employing best practices in proposal preparation, researchers can significantly improve their chances of obtaining timely endorsements while prioritizing participant welfare.
This article outlines key steps to navigate the complexities of clinical research effectively. Emphasizing the importance of clear objectives, ethical principles, and meticulous documentation ensures that researchers can meet the diverse requirements of various ethics boards. Furthermore, the proactive communication strategies highlighted facilitate smoother interactions and expedite the approval process, ultimately benefiting patient access to innovative therapies.
In light of these insights, it is crucial for researchers to embrace the dual submission approach and adhere to ethical guidelines diligently. By doing so, they not only contribute to the advancement of medical science but also uphold the trust and safety of participants involved in their studies. Engaging with comprehensive clinical trial management services can further support researchers in overcoming potential challenges, ensuring that ethical integrity remains at the forefront of their endeavors.
Why is it important to clarify aims in clinical research?
Clearly specifying the objectives of a project is essential in medical research to define primary objectives, such as assessing the efficacy of a new treatment or understanding a disease's progression.
What ethical considerations should be integrated into clinical research?
Ethical considerations must prioritize the rights and welfare of participants. Researchers should refer to established ethical frameworks, such as the Belmont Report, which outlines principles like respect for persons, beneficence, and justice.
How can researchers ensure their aims align with ethical principles?
Researchers can promote trust and integrity in their work by aligning research aims with ethical principles outlined in frameworks such as the Belmont Report.
What services does bioaccess provide to support ethical considerations in clinical research?
Bioaccess provides comprehensive clinical trial management services, including feasibility analyses, selection of research locations and principal investigators, compliance assessments of research documents, and management of import permits and nationalization of investigational devices.
How does bioaccess facilitate ethical trial setup and approval processes?
Bioaccess facilitates ethical trial setup and approval processes by ensuring compliance with country requirements and assisting with the approval processes through ethics committees and health ministries.
What are the key steps to follow in clinical research regarding aims and ethics?
The key steps include defining research objectives, incorporating ethical principles to respect participant rights and welfare, and consulting ethical guidelines like the Belmont Report to guide ethical considerations.