


Mastering the complexities of the ALIMS regulatory framework is crucial for biopharma companies that aspire to excel in a competitive environment. This guide explores the essential elements of ALIMS inspection readiness, providing insights that can significantly bolster compliance and operational success. With regulations constantly evolving and the intricacies of clinical trials at play, organizations must ask: how can they effectively navigate these challenges to meet the rigorous standards established by ALIMS?
By understanding the nuances of ALIMS, companies can position themselves not just to comply, but to thrive. This guide will delve into the critical components of inspection readiness, highlighting strategies that enhance both compliance and operational efficiency. As we explore these insights, consider how your organization can leverage this knowledge to overcome the hurdles in clinical research.
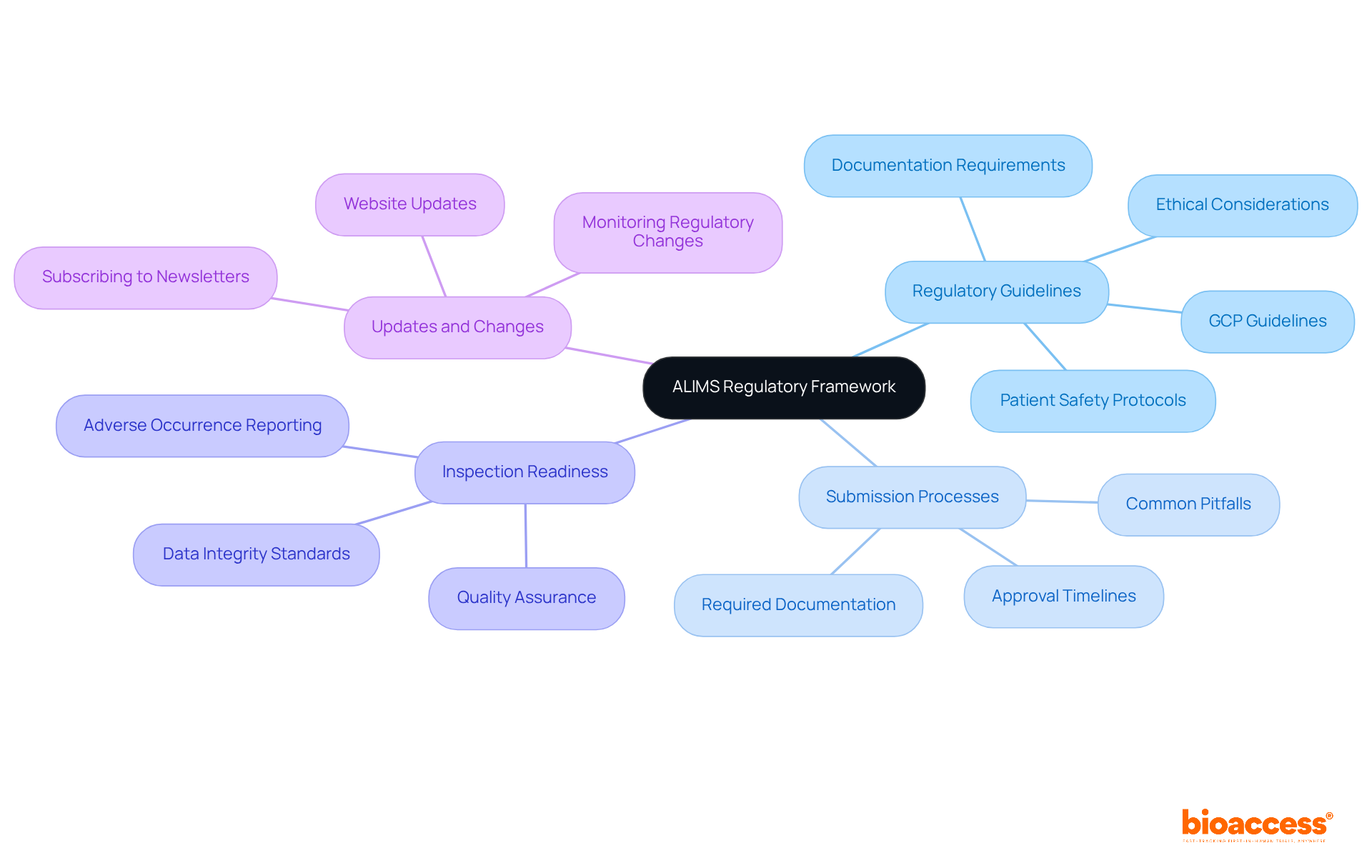
The regulatory framework established by the Agency for Medicines and Medical Devices of Serbia is crucial for the approval and oversight of clinical trials in the country. Understanding the following key components is essential for ensuring compliance and achieving success in your clinical research endeavors:
Regulatory Guidelines: Reviewing the latest ALIMS guidelines on Good Clinical Practice (GCP) and related regulations is vital. A thorough understanding of documentation requirements, ethical considerations, and patient safety protocols forms the foundation for maintaining high standards in clinical research.
Submission Processes: Grasping the submission processes for clinical study applications is essential. This includes timelines, required documentation, and common pitfalls that could lead to delays in approval. Recent statistics show that most clinical study applications receive approval well within a 60-day review period, highlighting the system's efficiency when navigated correctly.
Familiarity with the ALIMS inspection readiness for biopharma compliance standards is necessary. This encompasses expectations for data integrity, timely reporting of adverse occurrences, and sustaining quality assurance throughout the study. Adhering to these standards not only ensures regulatory compliance but also enhances your ALIMS inspection readiness for biopharma research credibility.
Updates and Changes: Staying informed about recent alterations to regulations or procedures is essential for any clinical study sponsor. Regularly monitoring the designated website and subscribing to relevant newsletters can provide timely updates that may influence your trial, ensuring compliance with the latest requirements.
By mastering these elements, you will be better prepared for evaluations and can ensure adherence throughout your clinical research activities. This ultimately contributes to the success of your biopharma initiatives.
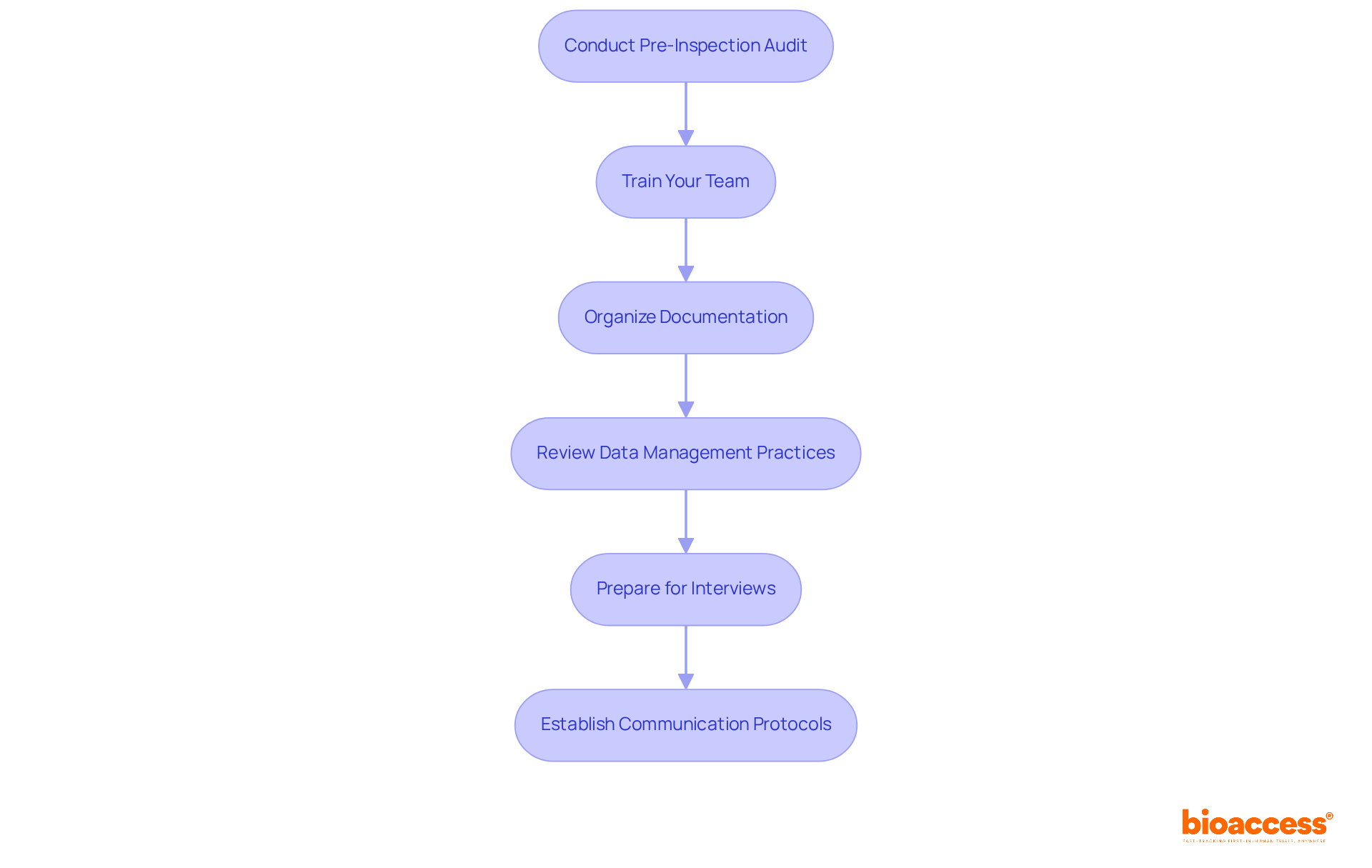
To effectively prepare for an ALIMS inspection, follow these essential steps:
Conduct a Pre-Inspection Audit: Start with an internal audit of your clinical trial documentation and processes. Evaluate for thoroughness, precision, and adherence to regulations. This crucial step can significantly enhance ALIMS inspection readiness for biopharma by reducing common shortcomings found during evaluations.
Train your team to ensure that all members are well-versed in ALIMS inspection readiness for biopharma and understand their specific roles during the evaluation. Conduct mock evaluations to replicate the actual process, fostering familiarity and confidence among staff.
Organize Documentation: Compile all essential documents, including trial protocols, informed consent forms, and safety reports. Make sure these documents are easily accessible and systematically organized to facilitate a smooth inspection process.
Review data management practices to confirm they meet the requirements for ALIMS inspection readiness for biopharma. This includes ensuring data accuracy, completeness, and secure storage, which are vital for meeting regulations.
Prepare for Interviews: Anticipate potential questions from inspectors and prepare your team to respond effectively. This preparation should include a thorough understanding of the rationale behind study protocols and patient safety measures.
Establish Communication Protocols: Designate a primary point of contact for the assessment and ensure all team members are informed on effective communication strategies during the process.
By implementing these measures, you can significantly enhance your preparedness for a regulatory inspection, showcasing your commitment to adherence and operational excellence.
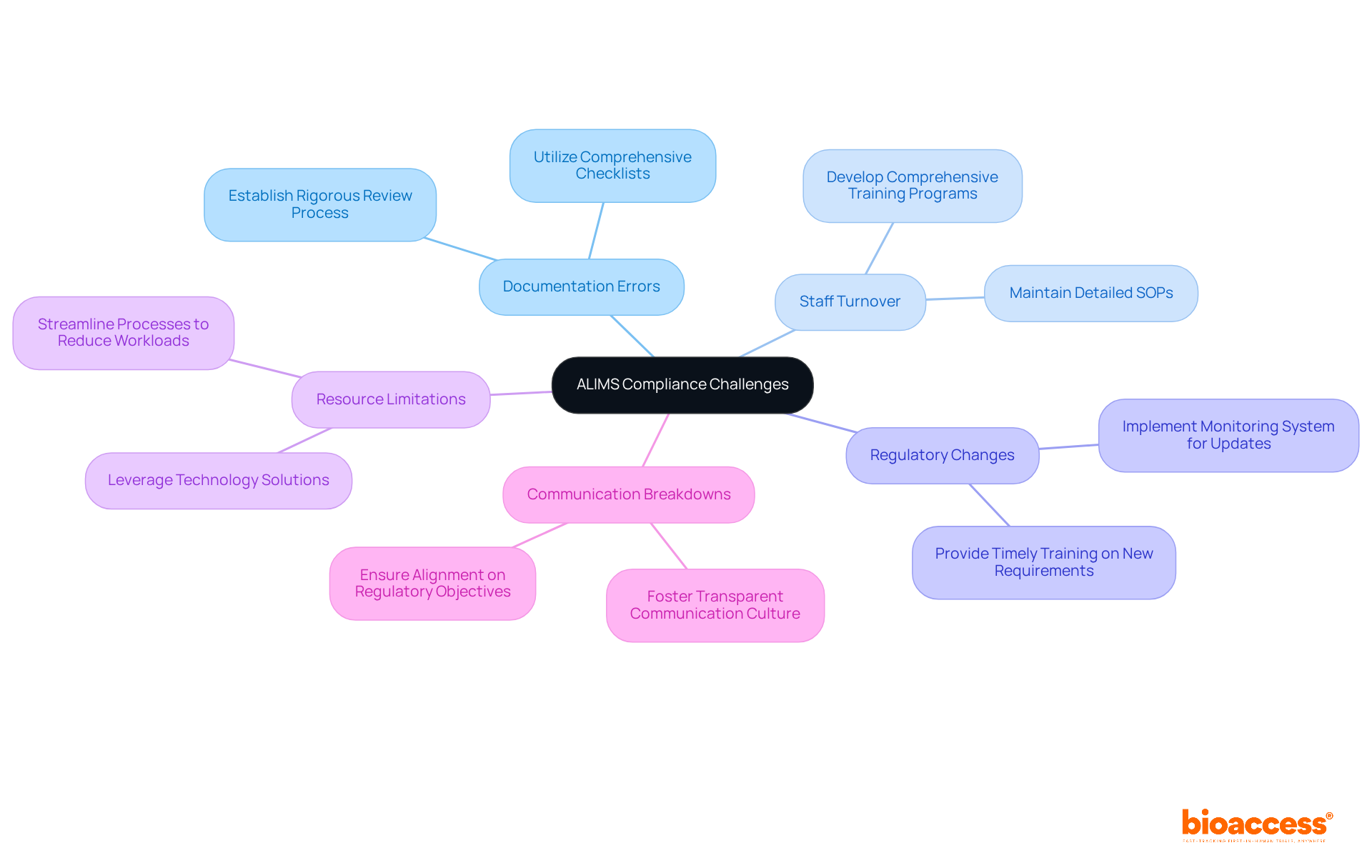
Compliance with ALIMS regulations presents several significant challenges that demand strategic solutions. Understanding these issues is crucial for organizations aiming to enhance their compliance posture in the clinical research landscape. Here are key challenges and effective strategies to address them:
Documentation Errors: Inaccurate or incomplete documentation often leads to regulatory issues. To combat this, establish a rigorous documentation review process and utilize comprehensive checklists to ensure all necessary documents are complete and accurate.
Staff Turnover: High turnover rates can create substantial knowledge gaps regarding adherence protocols. To mitigate this risk, develop comprehensive training programs and maintain detailed Standard Operating Procedures (SOPs) that are readily accessible to all team members, ensuring continuity in regulatory practices.
Regulatory Changes: The regulatory landscape is constantly evolving, making compliance a moving target. Implement a robust system for monitoring regulatory updates and ensure your team receives timely training on any new requirements as they arise.
Resource Limitations: Constraints in resources can impede adherence efforts. Leverage technology solutions for data management and regulatory tracking to streamline processes, reduce manual workloads, and enhance overall efficiency.
Communication Breakdowns: Ineffective communication can lead to misunderstandings and adherence failures. Foster a culture of transparent communication within your team and with external stakeholders to ensure alignment on regulatory objectives and promote a collaborative atmosphere.
By proactively addressing these challenges, organizations can fortify their compliance posture and enhance alims inspection readiness for biopharma.
Mastering ALIMS inspection readiness is not just beneficial; it is essential for biopharma success, directly influencing the approval and oversight of clinical trials. Organizations that thoroughly understand the regulatory framework, preparation processes, and compliance challenges significantly enhance their credibility and operational excellence in clinical research.
Key insights highlight the necessity of:
Moreover, addressing challenges such as documentation errors and staff turnover through strategic solutions can greatly improve compliance posture. By emphasizing effective communication and continuous training, organizations can ensure a robust approach to navigating the regulatory landscape.
The significance of ALIMS inspection readiness cannot be overstated. Organizations prioritizing compliance and preparation not only increase their chances of successful inspections but also contribute to the overall advancement of the biopharma industry. Embracing these practices paves the way for innovative solutions and improved patient outcomes, underscoring the vital role of regulatory adherence in achieving biopharma success.
What is the ALIMS regulatory framework?
The ALIMS regulatory framework, established by the Agency for Medicines and Medical Devices of Serbia, is crucial for the approval and oversight of clinical trials in the country.
Why is it important to review the ALIMS guidelines on Good Clinical Practice (GCP)?
Reviewing the latest ALIMS guidelines on GCP is vital for understanding documentation requirements, ethical considerations, and patient safety protocols, which are essential for maintaining high standards in clinical research.
What should I know about the submission processes for clinical study applications?
It is important to understand the submission processes, including timelines, required documentation, and common pitfalls that could lead to approval delays. Most clinical study applications receive approval within a 60-day review period if navigated correctly.
What does ALIMS inspection readiness entail?
ALIMS inspection readiness involves meeting biopharma compliance standards, which include expectations for data integrity, timely reporting of adverse occurrences, and maintaining quality assurance throughout the study.
How can I stay informed about updates and changes to regulations?
Staying informed about recent alterations to regulations or procedures is essential for any clinical study sponsor. Regularly monitoring the designated website and subscribing to relevant newsletters can provide timely updates that may influence your trial.
How does mastering the ALIMS regulatory framework contribute to clinical research success?
By mastering the key components of the ALIMS regulatory framework, sponsors will be better prepared for evaluations and ensure adherence throughout their clinical research activities, ultimately contributing to the success of their biopharma initiatives.