


Understanding the complexities of biosimilars is essential for navigating the evolving landscape of healthcare in Croatia. These innovative biological products not only enhance patient access to vital medications but also introduce significant regulatory challenges that companies must address. As the market for biosimilars expands, organizations face the pressing question: how can they effectively comply with the intricate naming and labeling rules set forth by regulatory bodies? This guide delves into the critical aspects of biosimilar regulations in Croatia, offering insights and strategies to streamline the approval process and ensure successful market entry.
Biosimilars represent a pivotal advancement in the realm of biological substances, demonstrating a high degree of similarity to already approved reference items in terms of quality, safety, and efficacy. Unlike generics, which are exact replicas of chemical medications, biosimilars are inherently different due to the complex nature of biological products.
The introduction of these similar biological products in the country is particularly significant; it not only enhances patient access to essential medications but also stimulates competition within the healthcare market.
Understanding the meaning and importance of biosimilars is the crucial first step in navigating the compliance landscape effectively.
In Croatia, the biosimilar naming and labeling rules in Croatia are shaped by the Agency for Medicinal Products and Medical Devices (HALMED) and the European Medicines Agency (EMA) in the regulatory landscape for similar biological products. Companies must demonstrate biosimilarity to a reference product through rigorous analytical, preclinical, and clinical studies as part of the approval process. This includes a centralized marketing authorization, essential for ensuring that similar biological products adhere to the stringent EU standards for quality, safety, and efficacy. As we approach 2025, understanding and adhering to the biosimilar naming and labeling rules in Croatia is essential for navigating the complexities of biosimilar approvals.
Companies like bioaccess® have adeptly navigated these regulations, underscoring the importance of a thorough understanding of the regulatory environment. With over 50 pre-qualified sites activated in less than eight weeks, bioaccess® accelerates clinical trials through its comprehensive services, ensuring compliance with FDA, EMA, and MDR standards. Expert insights reveal that familiarity with the biosimilar naming and labeling rules in Croatia, along with HALMED's specific requirements, can significantly streamline the approval process, ultimately facilitating quicker market access for innovative biosimilar therapies.
In this evolving Medtech landscape, collaboration and expertise are paramount. By leveraging established knowledge and resources, companies can effectively address the challenges of clinical research and regulatory compliance.
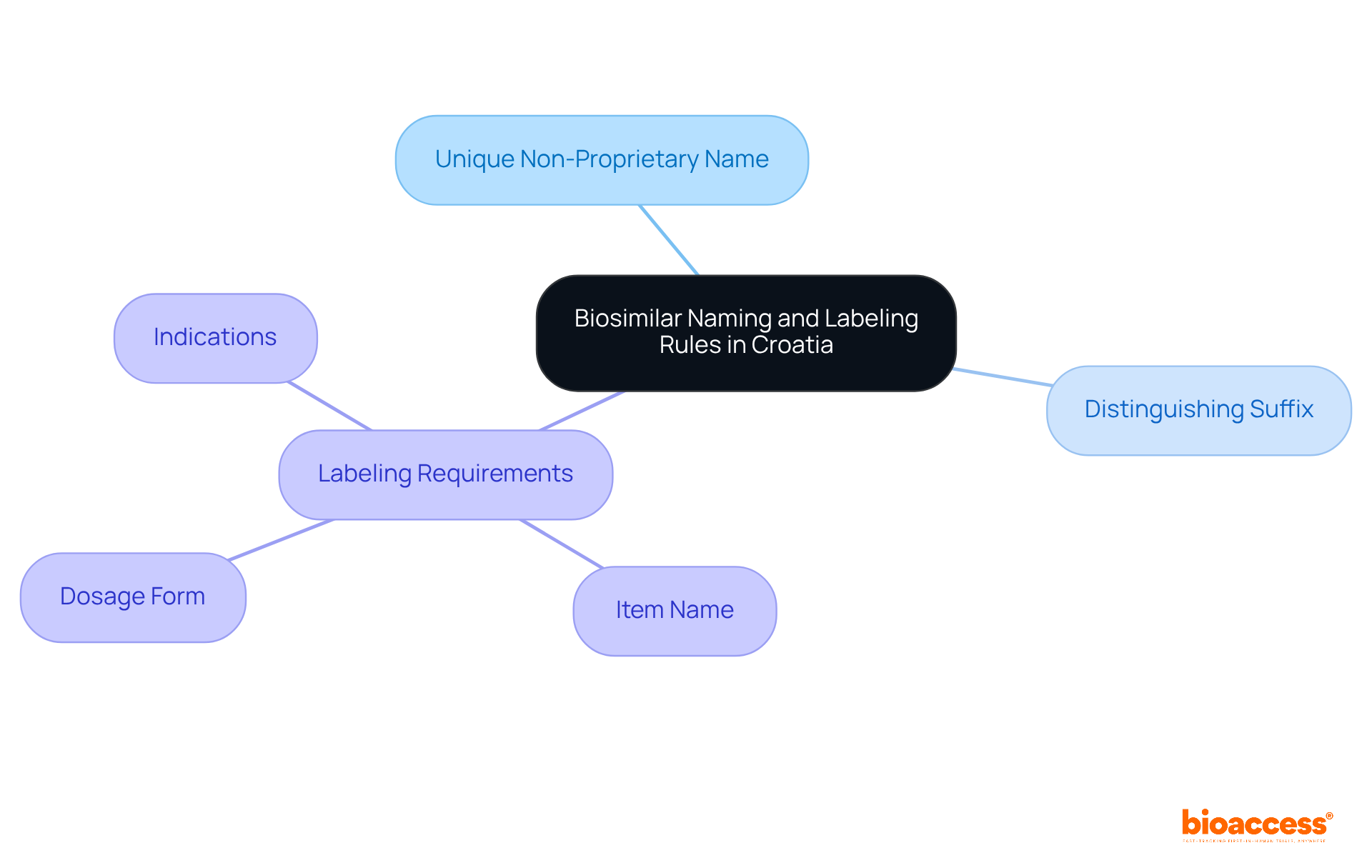
In Croatia, the biosimilar naming and labeling rules in Croatia mandate that biosimilars follow specific naming conventions set forth by the EMA. According to the biosimilar naming and labeling rules in Croatia, each biosimilar must have a unique non-proprietary name that includes a distinguishing suffix, which is essential to prevent confusion with the reference item. Additionally, all labeling must conform to the biosimilar naming and labeling rules in Croatia and must include critical information such as:
Ensuring that all labeling complies with the biosimilar naming and labeling rules in Croatia, as well as local and EU regulations, is crucial for facilitating market entry and maintaining transparency with healthcare professionals and patients.
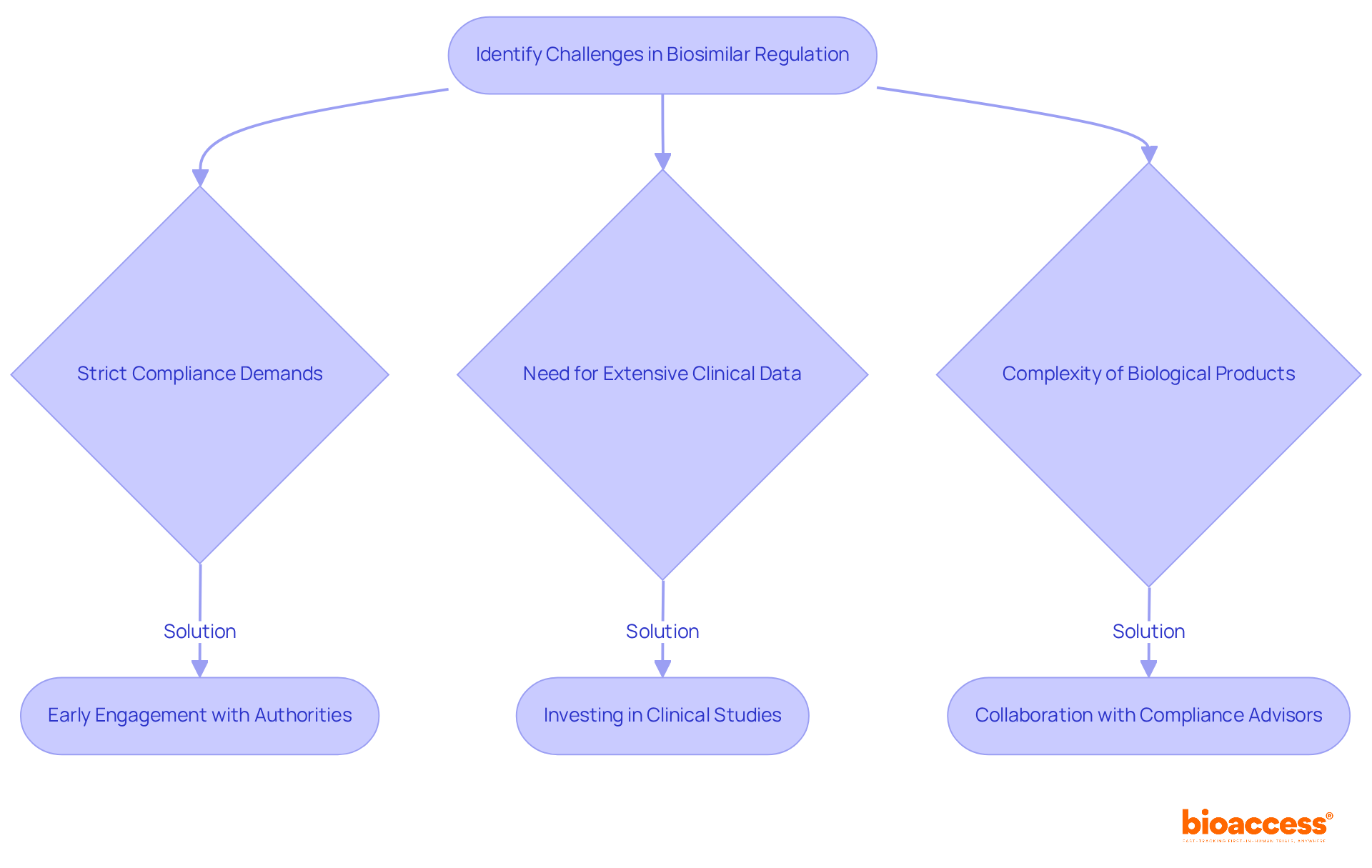
The rise of the biosimilar market in Croatia presents significant opportunities as well as notable challenges related to biosimilar naming and labeling rules in Croatia. Companies face strict compliance demands and the necessity for extensive clinical data, making the demonstration of biosimilarity particularly complex due to the intricate nature of biological products. To effectively navigate these hurdles, organizations must prioritize early engagement with oversight authorities throughout the development process.
Investing in robust clinical studies is essential, as is staying informed about evolving regulations that could impact compliance. Collaborating with knowledgeable compliance advisors can provide critical insights and streamline the adherence process, ultimately facilitating smoother market entry. Statistics reveal that regulatory compliance issues frequently hinder progress, with many companies experiencing delays in approval timelines.
By adopting these proactive strategies, organizations can significantly enhance their chances of successful biosimilar commercialization in Croatia while adhering to the biosimilar naming and labeling rules in Croatia. The importance of collaboration and strategic planning cannot be overstated; these elements are vital for overcoming the challenges that lie ahead.
Understanding the complexities of biosimilar naming and labeling rules in Croatia is crucial for navigating the intricate landscape of biological products. These regulations are not just bureaucratic hurdles; they are essential components that ensure the safety, efficacy, and accessibility of biosimilars within the healthcare system. By mastering these guidelines, companies can refine their market entry strategies and ultimately contribute to better patient outcomes.
The article underscores several key points:
Given these insights, it is imperative for stakeholders in the biosimilar market to prioritize compliance and collaboration. By investing in knowledge and resources, companies can not only streamline their approval processes but also play a pivotal role in shaping a more competitive and accessible healthcare environment in Croatia. Embracing these regulations transcends mere legal compliance; it fosters innovation and enhances the lives of patients who rely on these therapies.
What are biosimilars?
Biosimilars are biological substances that demonstrate a high degree of similarity to already approved reference products in terms of quality, safety, and efficacy.
How do biosimilars differ from generics?
Unlike generics, which are exact replicas of chemical medications, biosimilars are inherently different due to the complex nature of biological products.
Why are biosimilars important in Croatia?
The introduction of biosimilars in Croatia enhances patient access to essential medications and stimulates competition within the healthcare market.
What is the first step in navigating the compliance landscape for biosimilars?
Understanding the meaning and importance of biosimilars is the crucial first step in navigating the compliance landscape effectively.