


Australia's biopharma sector stands at a pivotal moment, brimming with potential thanks to a supportive economic environment and an efficient regulatory framework that fosters innovation. This article explores the complexities of budgeting biopharma trials within this dynamic landscape, shedding light on cost components and strategic advantages that can yield substantial savings. As companies navigate these financial waters, they must confront a crucial question: how can they effectively leverage local resources and foster stakeholder collaboration to optimize their trial budgets and enhance research outcomes?
By addressing this question, we aim to provide valuable insights that not only highlight the importance of collaboration but also outline actionable steps for companies to take in order to thrive in this competitive field.
Australia's biopharma landscape presents a compelling opportunity for growth, characterized by a favorable economic environment and a streamlined regulatory framework. The government actively supports innovation through substantial financial incentives, such as the R&D Tax Incentive, which offers rebates of up to 43.5% on eligible R&D expenditures. This significant support alleviates the financial burden on companies engaged in research studies. Additionally, the Therapeutic Goods Administration (TGA) oversees research approval processes, ensuring compliance with safety and effectiveness standards while facilitating quicker study initiation through its dual-track approval system.
In this dynamic environment, bioaccess® enhances the landscape by offering extensive clinical study oversight services. These include:
With a commitment to accelerating site activation in under eight weeks and a proven track record in navigating regulatory requirements across LATAM, the Balkans, and Australia, bioaccess is uniquely positioned to assist biopharma companies in budgeting biopharma trials in the Australian market while optimizing their budgets and timelines effectively.
The collaboration between biopharma companies and bioaccess is crucial for overcoming the challenges in clinical research. By leveraging bioaccess's expertise, companies can not only streamline their processes but also enhance their research outcomes. As the biopharma sector continues to evolve, the importance of such partnerships cannot be overstated. Companies are encouraged to consider how they can benefit from these services and take the next steps toward successful clinical research.
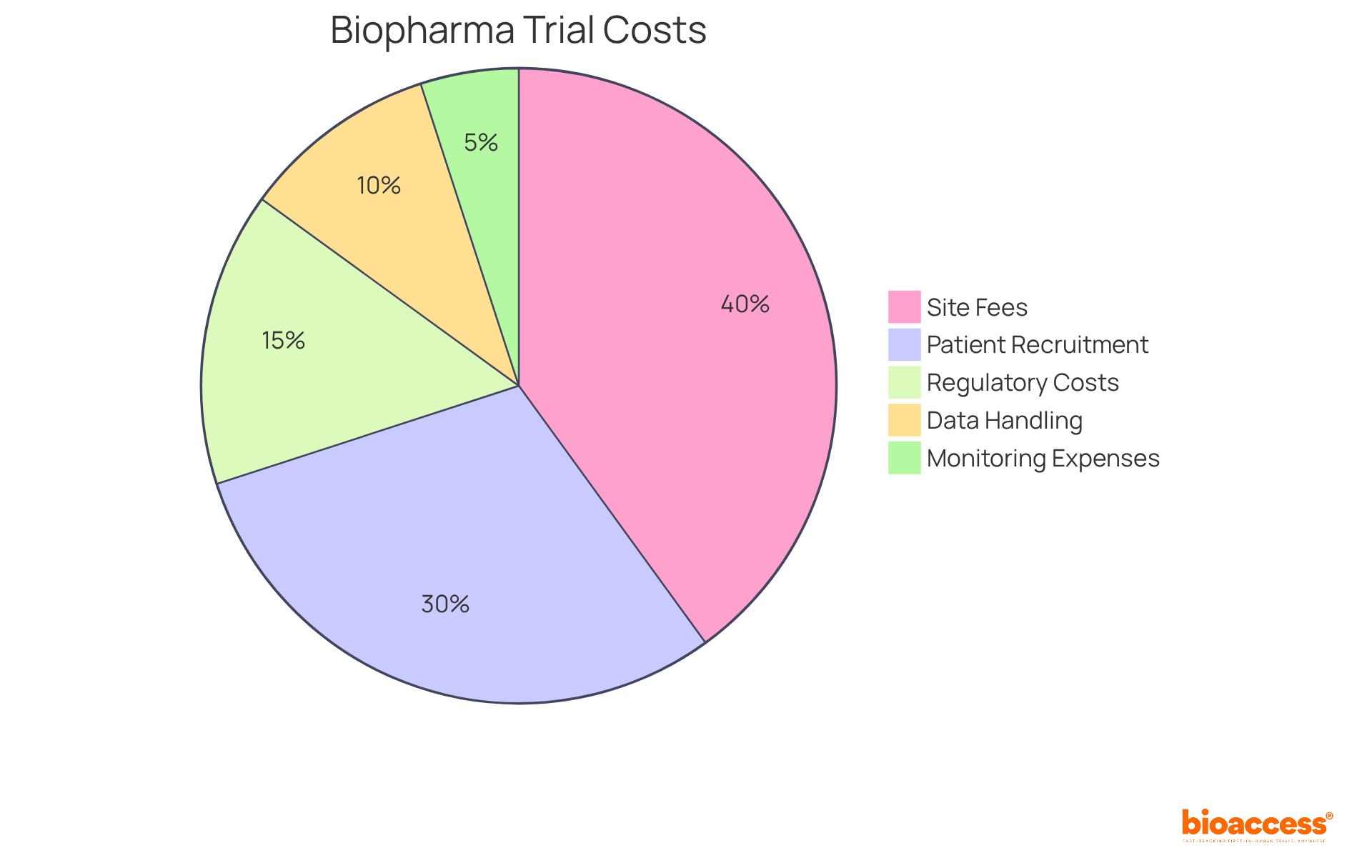
Budgeting biopharma trials in the Australian market requires a well-organized study budget that is crucial in clinical research, encompassing several essential elements:
Site oversight typically constitutes a substantial portion of the budget, often accounting for 30% to 50% of total expenses. In 2025, budgeting biopharma trials in the Australian market reflects these trends, underscoring the necessity for meticulous planning, especially since research starts in Australia can be up to 4-6 months quicker than in the US.
Patient recruitment is another vital area; delays in this phase can significantly inflate costs. Recruitment challenges can lead to financial burdens that may reach up to $8 million per month, as highlighted in industry reports. Regulatory adherence expenses, which include ethics committee fees and Therapeutic Goods Administration (TGA) submissions, are also critical to consider. By utilizing Bioaccess's extensive research study management services - including feasibility studies, site selection, compliance reviews, setup, import permits, project management, and reporting - biopharma companies can enhance their efficiency in budgeting biopharma trials in the Australian market.
This strategic approach not only improves financial planning but also enhances operational efficiency, ensuring a smoother path to regulatory approval and successful execution of the study. Collaboration with experts like Bioaccess can make a significant difference in navigating these complexities.
Biopharma firms can significantly enhance their capabilities in budgeting biopharma trials in the Australian market by tapping into local advantages, such as its diverse patient population and competitive medical service costs. Did you know that budgeting biopharma trials in the Australian market can be up to 28% less expensive than conducting early-phase studies in the United States before tax benefits? After factoring in tax benefits, the savings can soar to as much as 60%. This cost-effectiveness is enhanced by a streamlined regulatory environment, overseen by the Therapeutic Goods Administration (TGA), which supports budgeting biopharma trials in the Australian market through quicker approvals via the Clinical Trial Notification (CTN) scheme. This efficiency can drastically reduce time-to-market and associated costs.
Moreover, budgeting biopharma trials in the Australian market by utilizing local sites for patient recruitment not only enhances enrollment rates but also reduces travel expenses. By strategically partnering with knowledgeable local CROs, like bioaccess, companies can navigate the complexities of clinical studies more effectively. Bioaccess offers a comprehensive suite of research study management services, including:
This approach is particularly advantageous as we look toward 2025, with the demand for innovative therapies on the rise, positioning Australia as a premier destination for clinical research.
Effective cooperation with local stakeholders, such as healthcare providers, regulatory bodies, and patient advocacy groups, is crucial for enhancing budgeting biopharma trials in the Australian market. Engaging these stakeholders early in the planning process not only aligns expectations but also fosters resource sharing. For instance, local healthcare providers can significantly aid in patient recruitment and retention, while regulatory bodies provide essential guidance on adherence requirements.
bioaccess® stands out by offering comprehensive clinical study management services, including feasibility evaluations, site selection, compliance assessments, setup, import permits, project management, and reporting. Establishing robust relationships with these entities facilitates smoother trial operations and enables better negotiation terms, which is crucial for budgeting biopharma trials in the Australian market and ultimately reduces costs associated with trial execution.
In the evolving Medtech landscape, collaboration is not just beneficial; it’s essential. By leveraging the expertise of local stakeholders, biopharma companies can navigate challenges more effectively, which is crucial for budgeting biopharma trials in the Australian market and enhancing the overall success of their clinical trials. The next step is to actively engage with these partners to maximize the potential of your studies.
Navigating the complexities of budgeting biopharma trials in the Australian market is crucial for companies looking to optimize their financial resources and operational efficiency. Understanding the economic and regulatory landscape is not just important; it presents unique opportunities for innovation and cost savings. By leveraging local advantages and collaborating with experienced partners like bioaccess, biopharma companies can streamline their trial processes and significantly enhance their overall success.
Key insights reveal critical cost components that require meticulous planning, including:
The potential for substantial savings-up to 60% when considering tax benefits-highlights the necessity of strategic budgeting and local partnerships. Moreover, engaging with local stakeholders not only aligns expectations but also fosters resource sharing, which enhances the efficiency of trial execution.
As the demand for innovative therapies continues to rise, the Australian market emerges as a premier destination for clinical research. Biopharma companies are urged to embrace these insights and actively engage with local stakeholders to maximize their trial budgeting efforts. By doing so, they can mitigate costs and pave the way for successful and timely research outcomes, ultimately contributing to the advancement of healthcare.
What is the economic environment for biopharma trials in Australia?
Australia offers a favorable economic environment for biopharma trials, supported by substantial financial incentives such as the R&D Tax Incentive, which provides rebates of up to 43.5% on eligible R&D expenditures.
How does the regulatory framework in Australia support biopharma research?
The regulatory framework in Australia is streamlined, with the Therapeutic Goods Administration (TGA) overseeing research approval processes to ensure compliance with safety and effectiveness standards while facilitating quicker study initiation through a dual-track approval system.
What services does bioaccess® provide for clinical studies?
bioaccess® offers extensive clinical study oversight services including feasibility assessments, site selection, adherence reviews, setup, import permits, project coordination, and reporting.
How quickly can bioaccess® activate study sites?
bioaccess® is committed to accelerating site activation in under eight weeks.
What experience does bioaccess® have in navigating regulatory requirements?
bioaccess® has a proven track record in navigating regulatory requirements across LATAM, the Balkans, and Australia, making it well-equipped to assist biopharma companies.
Why is collaboration with bioaccess® important for biopharma companies?
Collaboration with bioaccess® helps biopharma companies overcome challenges in clinical research, streamline their processes, and enhance research outcomes.
What should biopharma companies consider when planning their research in Australia?
Companies are encouraged to consider how they can benefit from the services provided by bioaccess® to optimize their budgets and timelines effectively for successful clinical research.