


The article emphasizes crucial strategies for ensuring compliance and data integrity in clinical testing, underscoring the significance of regulatory adherence and robust data management practices. It highlights the necessity of following established guidelines, such as:
These elements collectively enhance the credibility and safety of clinical trials, reinforcing the importance of a structured approach in the Medtech landscape.
Clinical testing stands as a cornerstone of modern medicine, meticulously designed to evaluate the safety and efficacy of new medical interventions. These trials form the foundation of evidence-based healthcare, making it crucial for researchers and organizations to grasp the intricacies of compliance and data integrity.
What strategies can be employed to navigate the complex regulatory landscape and ensure robust data management? This article delves into essential best practices that not only enhance compliance but also fortify the integrity of clinical research. Ultimately, these practices lead to improved patient outcomes and innovative treatments.
Clinical testing represents systematic investigations meticulously designed to assess the safety and efficacy of new medical interventions, which include drugs, devices, and treatment protocols. These studies are crucial for advancing medical knowledge and enhancing patient care. By rigorously evaluating new therapies within regulated settings, clinical testing helps determine the effectiveness and safety of these interventions for public use.
The significance of research studies, including clinical testing, is profound; they form the bedrock of evidence-based medicine, providing the essential information required for regulatory approvals and the establishment of medical guidelines. For instance, the successful conclusion of a medical study can lead to the endorsement of a new cancer therapy, significantly influencing patient outcomes and survival statistics.
Furthermore, research studies contribute to understanding disease mechanisms and the development of innovative treatments, ultimately elevating the quality of healthcare and broadening access.
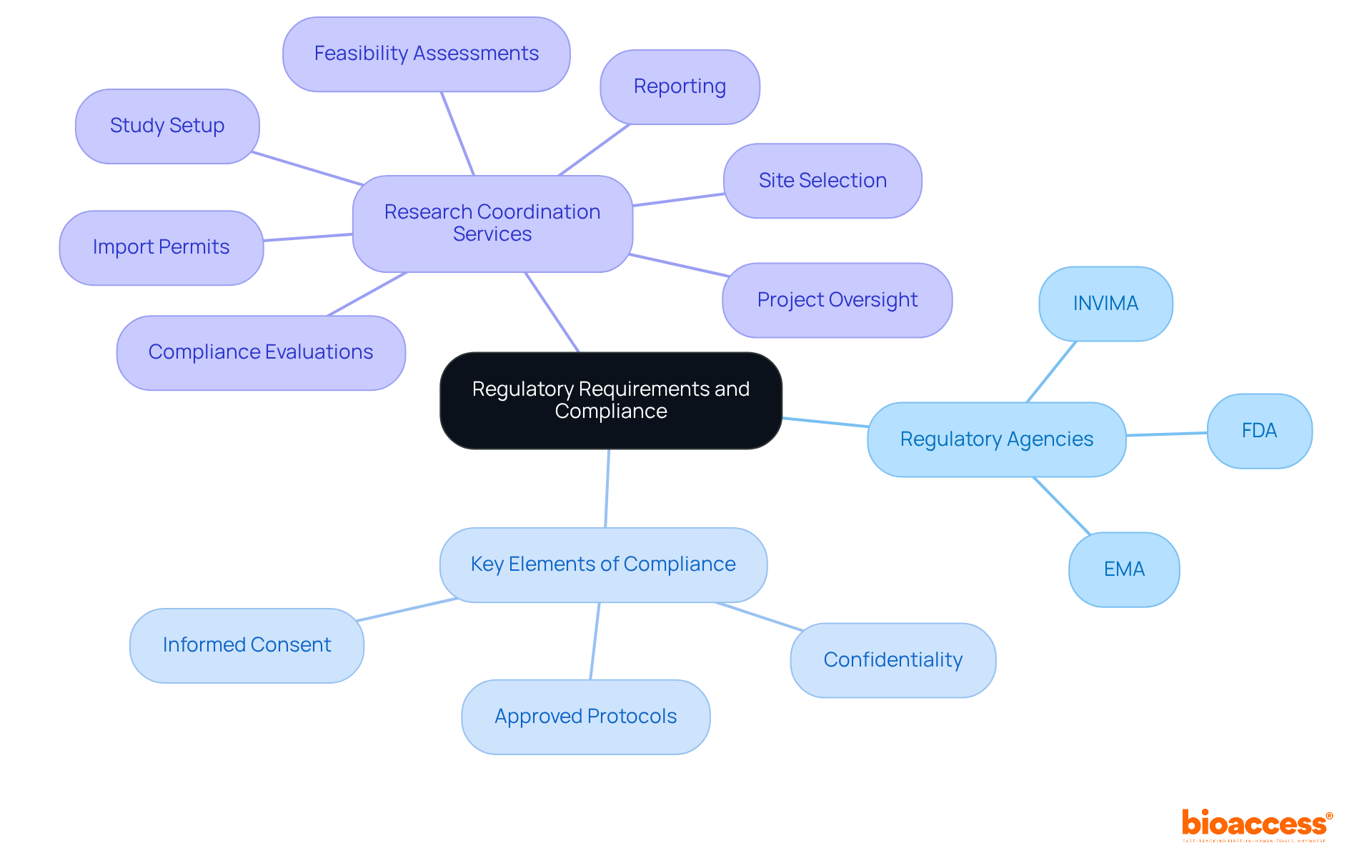
Navigating the regulatory landscape is a critical element of conducting clinical testing, particularly in the realm of medical devices. Regulatory agencies, such as the FDA in the United States and the EMA in Europe, establish guidelines that safeguard the safety and rights of participants in these studies. In Colombia, this process involves obtaining approvals from the ethics committee (IRB/EC), INVIMA, and securing the MinCIT import permit for investigational devices. Compliance with these regulations is not merely a legal obligation; it is a moral imperative.
Key elements of regulatory adherence include:
For example, adherence to GCP guidelines ensures that studies are conducted ethically, yielding trustworthy and valid data. Organizations must remain vigilant about evolving regulations, such as the recent changes in the Clinical Trials Regulation in the EU, which aim to streamline processes while enhancing participant protection.
By prioritizing adherence and leveraging comprehensive research study coordination services—such as feasibility assessments, site selection, compliance evaluations, study setup, import permits, project oversight, and reporting—researchers can mitigate risks and bolster the credibility of their findings. This holistic approach is essential for addressing the challenges encountered by medical device startups, including regulatory hurdles, competition, recruitment issues, and financial constraints.
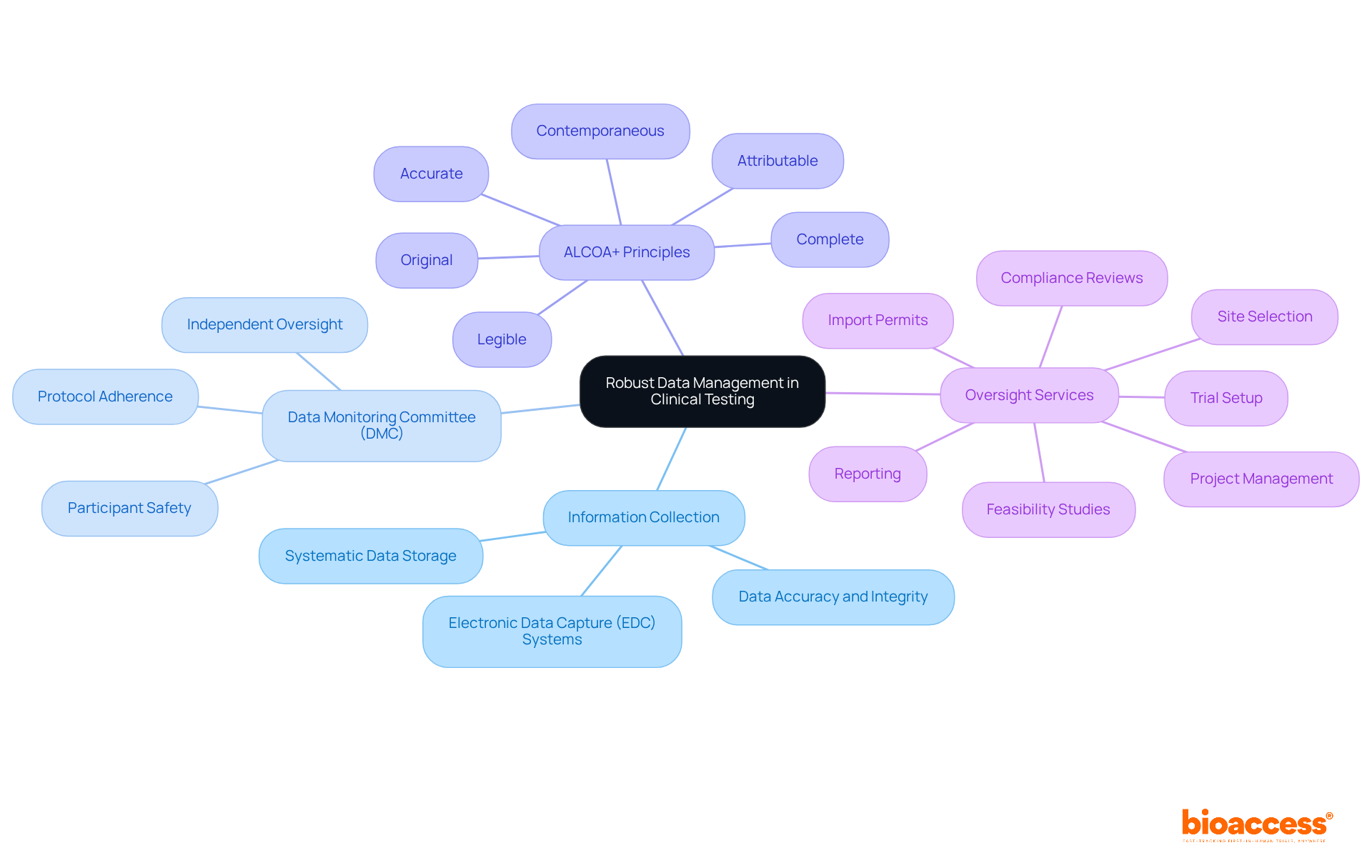
Strong information management practices are essential to the success of clinical testing. This involves the systematic collection, storage, and analysis of information to ensure its accuracy and integrity. Implementing electronic information capture (EDC) systems can streamline information collection and reduce errors associated with manual entry. Furthermore, consistent oversight of information during the experiment is crucial to recognize and resolve inconsistencies or problems swiftly.
For instance, employing a Data Monitoring Committee (DMC) provides independent oversight, ensuring that the trial adheres to its protocol and prioritizes participant safety. Moreover, organizations should embrace the ALCOA+ principles (Attributable, Legible, Contemporaneous, Original, Accurate, and Complete) to maintain information integrity. By fostering a culture of quality and adherence in data oversight, researchers can improve the reliability of their findings and facilitate clinical testing for the successful progression of new therapies.
Utilizing extensive research study oversight services, such as those provided by bioaccess®, can further enhance these practices. Their expertise in feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting ensures meticulous management of all trial aspects, ultimately contributing to the success of clinical research initiatives.
In conclusion, the importance of compliance and data integrity in clinical testing cannot be overstated. As the landscape of clinical research continues to evolve, it is crucial for researchers and organizations to remain vigilant and proactive. By embracing best practices and fostering a culture of quality, the medical community can ensure that clinical trials not only meet regulatory standards but also contribute to the betterment of patient care and the advancement of medical knowledge.
What are clinical trials?
Clinical trials are systematic investigations designed to assess the safety and efficacy of new medical interventions, including drugs, devices, and treatment protocols.
Why are clinical trials important?
Clinical trials are important because they advance medical knowledge, enhance patient care, and provide critical information for regulatory approvals and the establishment of medical guidelines.
How do clinical trials contribute to evidence-based medicine?
Clinical trials form the foundation of evidence-based medicine by providing essential information that supports the approval of new therapies and informs medical guidelines.
What impact can successful clinical trials have on patient outcomes?
Successful clinical trials can lead to the endorsement of new therapies, significantly influencing patient outcomes and survival statistics, particularly in areas like cancer treatment.
In what ways do clinical trials help in understanding diseases?
Clinical trials contribute to a better understanding of disease mechanisms and facilitate the development of innovative treatments, ultimately improving healthcare quality and access.