


This article emphasizes the mastery of clinical trials data management as a cornerstone for research success. It underscores the necessity of regulatory compliance, efficient data practices, and the strategic use of technology. By establishing compliance with regulations such as Good Clinical Practice, implementing standardized data management protocols, and utilizing electronic data capture systems, the integrity and reliability of clinical research outcomes are significantly enhanced. This approach not only fosters trust in research findings but also aligns with industry standards, paving the way for impactful contributions to the Medtech landscape.
Navigating the complexities of clinical trials data management is critical for ensuring research success and regulatory compliance. As the landscape of clinical research evolves, understanding best practices and technological advancements becomes paramount for researchers aiming to enhance the quality and reliability of their findings. However, with increasing demands for data integrity and efficiency, organizations must consider how to effectively implement these strategies while overcoming potential barriers.
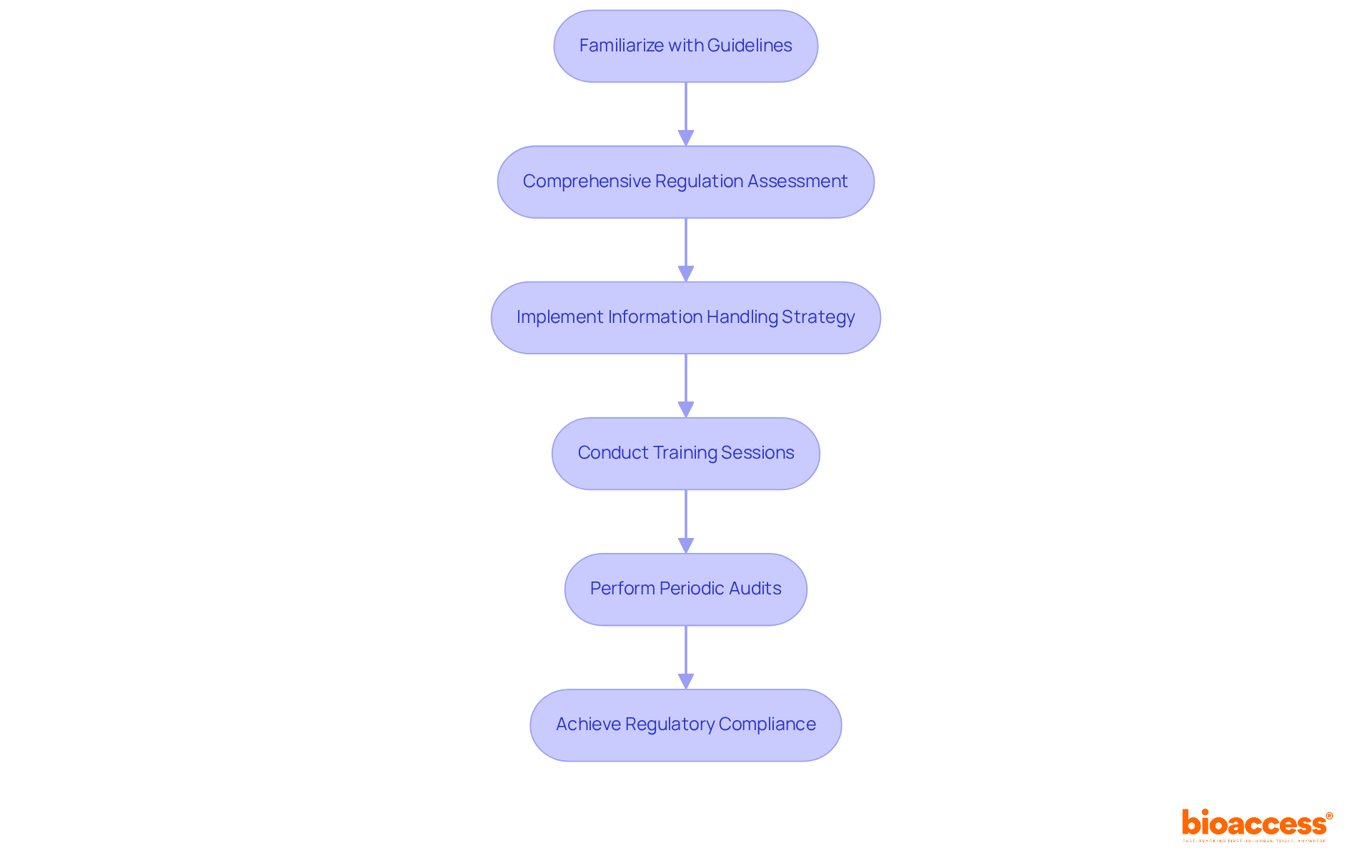
To establish regulatory compliance in information management, it is essential to familiarize yourself with relevant guidelines such as Good Clinical Practice (GCP) and local regulatory requirements. Begin with a comprehensive assessment of the regulations pertinent to your specific clinical study, including considerations for feasibility, research locations, and principal investigators (PIs). This process involves understanding the requirements for information collection, storage, and reporting, while ensuring adherence to study documents.
Next, implement a robust information handling strategy that outlines how information will be processed throughout the experiment. This plan should encompass protocols for information entry, validation, and audit trails to guarantee traceability. Regular training sessions for your team on compliance standards can further reinforce the importance of adhering to these regulations. Additionally, conducting periodic audits to assess compliance and identify areas for improvement is advisable.
By prioritizing regulatory compliance and utilizing clinical trials data management services—such as trial setup, import permits, project coordination, and reporting—you not only safeguard the integrity of your research but also enhance the credibility of your findings in the eyes of stakeholders and regulatory bodies.
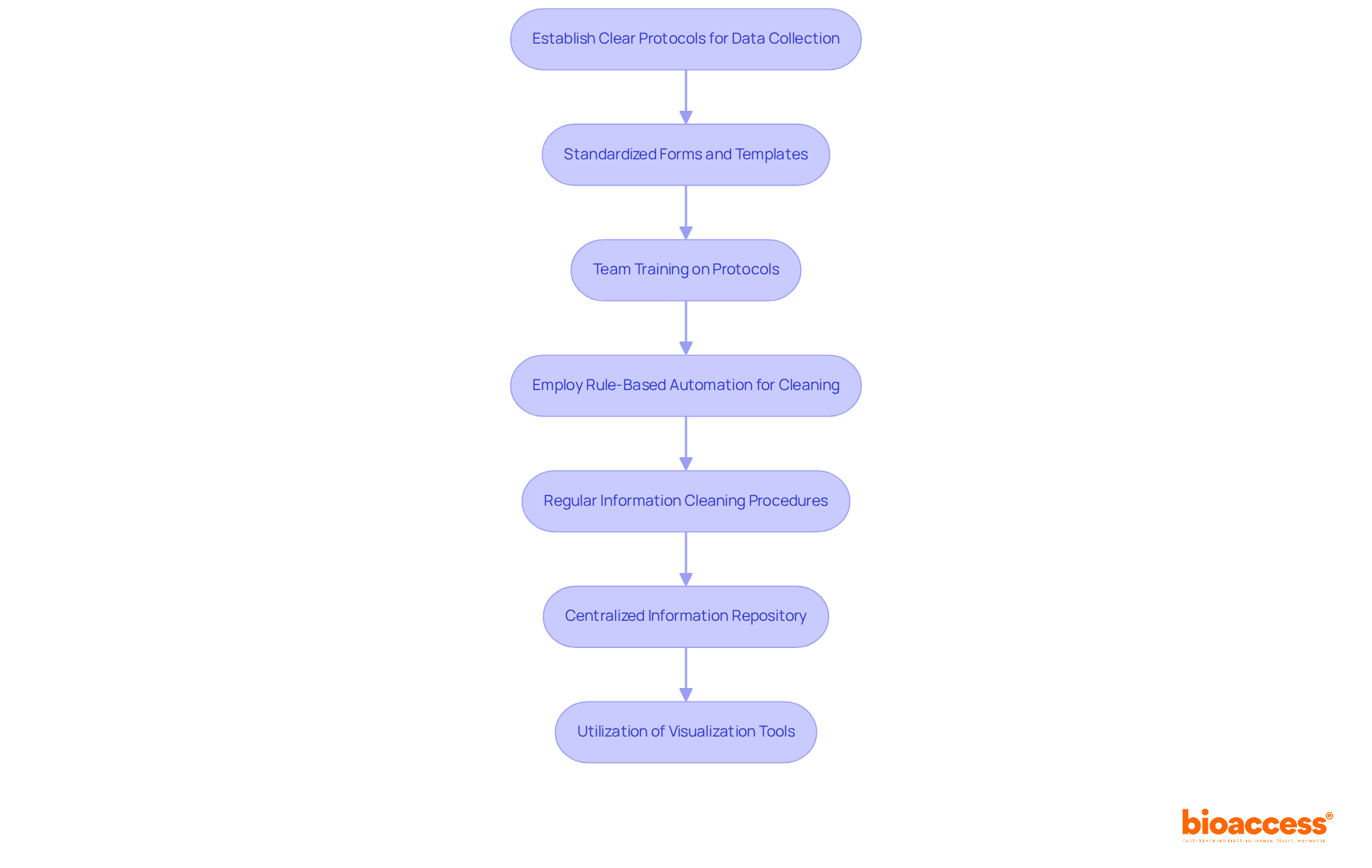
Implementing efficient clinical trials data management practices is essential for clinical research and begins with establishing clear protocols for data collection and entry. Standardized forms and templates are crucial for ensuring consistency across all information points. Training your team on these protocols is vital to minimize variability and errors. Biopharma firms such as GSK demonstrate that employing rule-based automation for information cleaning can significantly expedite the time to database lock, highlighting the importance of these practices in clinical research.
Regular information cleaning procedures should be integrated to detect and correct discrepancies early in the study. This proactive approach can avert larger issues later on. Additionally, adopting a centralized information repository allows for real-time access and updates, enhancing collaboration among team members. As emphasized by Leianne Ebert, Head of Clinical Information Operations, monitoring quality is essential for maintaining high standards in clinical trials data management.
Utilizing visualization tools can further enhance interpretation, aiding in the identification of trends and insights. For instance, the Indiana Department of Transportation (INDOT) effectively implemented standardized information gathering techniques in their culvert information collection initiative, which significantly improved asset oversight capabilities. By fostering a culture of efficiency in information management, you can substantially elevate the quality and reliability of your research outcomes.
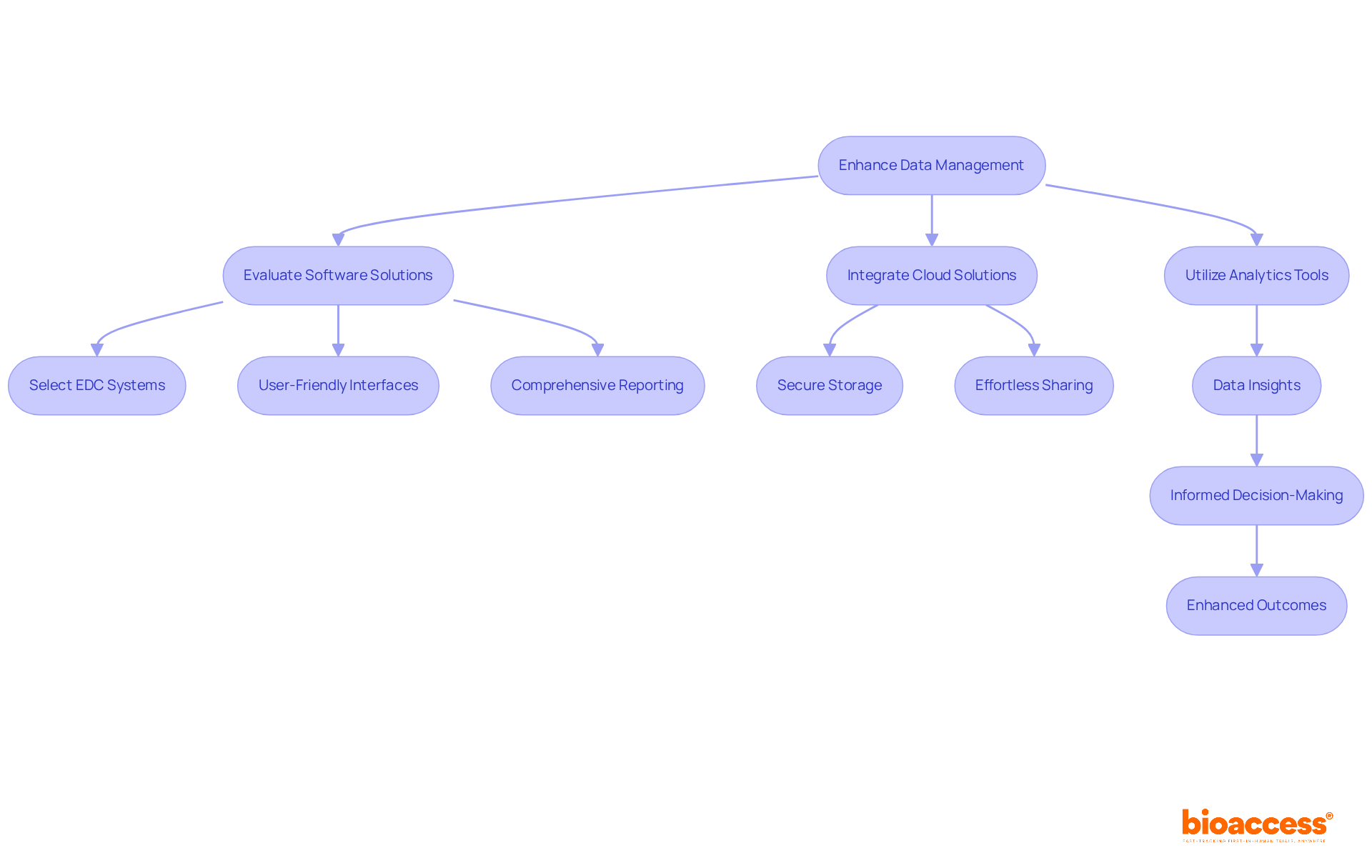
To enhance clinical trials data management, it is essential to evaluate software solutions specifically designed for clinical trials. Electronic Information Capture (EDC) systems play a crucial role in automating information collection, significantly minimizing manual entry mistakes. When selecting a platform, prioritize user-friendly interfaces and comprehensive reporting capabilities to streamline operations.
Integrating cloud-based solutions is vital for secure information storage and effortless sharing among team members, regardless of their geographical locations. This flexibility accelerates information access and improves collaboration among research teams.
Furthermore, utilizing analytics tools can yield significant insights from your data, uncovering patterns and correlations that might otherwise remain overlooked. Such insights are invaluable for informed decision-making, ultimately leading to enhanced outcomes in research studies. By embracing these technological advancements, you can revolutionize your data handling practices, paving the way for more successful research endeavors.
Notably, the use of EDC technologies can result in a 30% decrease in research timelines compared to conventional methods. As Jerry C Parker states, "The adoption of EDC technologies is transforming the landscape of medical studies." Moreover, the interoperability of EDC systems enhances information management by consolidating relevant details onto a single platform, which is crucial for maximizing efficiency.
However, it is important to acknowledge potential barriers to implementation, such as concerns regarding regulatory compliance, which can pose challenges in the adoption of these technologies. bioaccess® offers comprehensive clinical trials data management services, including:
to help navigate these challenges. Real-world examples of successful EDC implementation further illustrate their effectiveness, showcasing how organizations have benefited from these advancements.
Mastering clinical trials data management is vital for the success of research endeavors. Establishing regulatory compliance, implementing efficient data management practices, and leveraging advanced technologies are essential strategies that researchers must adopt to enhance the integrity and reliability of their findings while navigating the complexities of clinical trials.
The article underscores key strategies such as:
Each of these elements plays a crucial role in improving data accuracy and streamlining processes, ultimately contributing to more effective and credible research outcomes.
As the landscape of clinical research continues to evolve, embracing these best practices and technological advancements will not only improve operational efficiency but also ensure compliance with regulatory standards. Researchers are encouraged to adopt innovative tools and strategies to stay ahead in the rapidly changing field of clinical trials data management. This proactive approach will ultimately lead to more successful and impactful research initiatives.
What is the first step in establishing regulatory compliance in data management for clinical studies?
The first step is to familiarize yourself with relevant guidelines such as Good Clinical Practice (GCP) and local regulatory requirements, followed by a comprehensive assessment of the regulations pertinent to your specific clinical study.
What should be considered during the assessment of regulations for a clinical study?
Considerations should include feasibility, research locations, and principal investigators (PIs), as well as the requirements for information collection, storage, and reporting.
What is included in a robust information handling strategy?
A robust information handling strategy should outline how information will be processed throughout the experiment, including protocols for information entry, validation, and audit trails to ensure traceability.
How can a team reinforce adherence to compliance standards?
Regular training sessions for the team on compliance standards can reinforce the importance of adhering to these regulations.
Why is it advisable to conduct periodic audits?
Conducting periodic audits is advisable to assess compliance and identify areas for improvement.
What are the benefits of prioritizing regulatory compliance in clinical trials?
Prioritizing regulatory compliance safeguards the integrity of your research and enhances the credibility of your findings in the eyes of stakeholders and regulatory bodies.
What services can assist in clinical trials data management?
Clinical trials data management services can include trial setup, import permits, project coordination, and reporting.
10+ Data Governance Case Studies: Real-Life Examples (https://aimultiple.com/data-governance-case-studies)
Best Practices
Case Studies (https://ors.od.nih.gov/OD/OQM/benchmarking/bestpractice/Pages/case_studies.aspx)
Asset Management Field Data Collection Tools | GIS in Transportation | Planning, Environment & Realty | FHWA (https://gis.fhwa.dot.gov/case_studies/Asset_Management_Field_Data_Collection_Tools.aspx)
2025 Clinical Data Trend Report | Veeva (https://veeva.com/2025-clinical-data-trend-report)
Efficient Clinical Trial Data Management for Accurate Research (https://mindbowser.com/clinical-trial-data-management)