


Clinical project managers (CPMs) play a vital role in the success of clinical trials, overseeing project planning, budget management, regulatory compliance, and stakeholder communication throughout the trial process. Their critical role is underscored by the necessary skills they possess, the challenges they face, and the methodologies they employ to enhance project management efficiency. This highlights their importance in advancing medical research and improving patient care.
In the intricate world of clinical trials, the role of Clinical Project Managers (CPMs) is more critical than ever. As the demand for innovative medical solutions surges, these professionals stand at the forefront, orchestrating complex processes that ensure trials run smoothly and efficiently. This article delves into the essential practices, skills, and methodologies that empower CPMs to navigate challenges and enhance the success of clinical research.
What strategies can these managers adopt to overcome common obstacles and drive projects to completion in an ever-evolving landscape?
Project Managers (CPMs) play a crucial role in clinical trials project management, ensuring the successful execution of trials by overseeing the entire process from inception to completion. Their key responsibilities in clinical trials project management encompass:
Furthermore, in their clinical trials project management, they implement risk management strategies to proactively identify potential issues and safeguard the integrity of the trial, while encouraging cooperation among cross-functional teams to ensure alignment with objectives and timelines.
Statistics indicate that the demand for project managers in healthcare is projected to grow significantly, driven by an aging populace and advancing medical technologies. With an average salary of approximately $100,510, CPMs are well-compensated for their expertise, reflecting the substantial value assigned to their contributions in medical research. Their capability in clinical trials project management—covering multiple facets of a trial from participant selection to data analysis—underscores their pivotal role in advancing medical knowledge and enhancing patient care. Moreover, CPMs frequently utilize various templates and tools to boost productivity and ensure seamless execution.
At bioaccess®, the clinical trials project management (CPMs) team leverages extensive study coordination services, which include:
This comprehensive approach guarantees that assessments align with timelines and quality benchmarks while addressing the distinct challenges of the Latin American Medtech environment. Additionally, bioaccess®'s innovative solutions and efficient regulatory processes facilitate accelerated patient enrollment and regulatory approval, enabling faster access to groundbreaking medical technologies in the region.
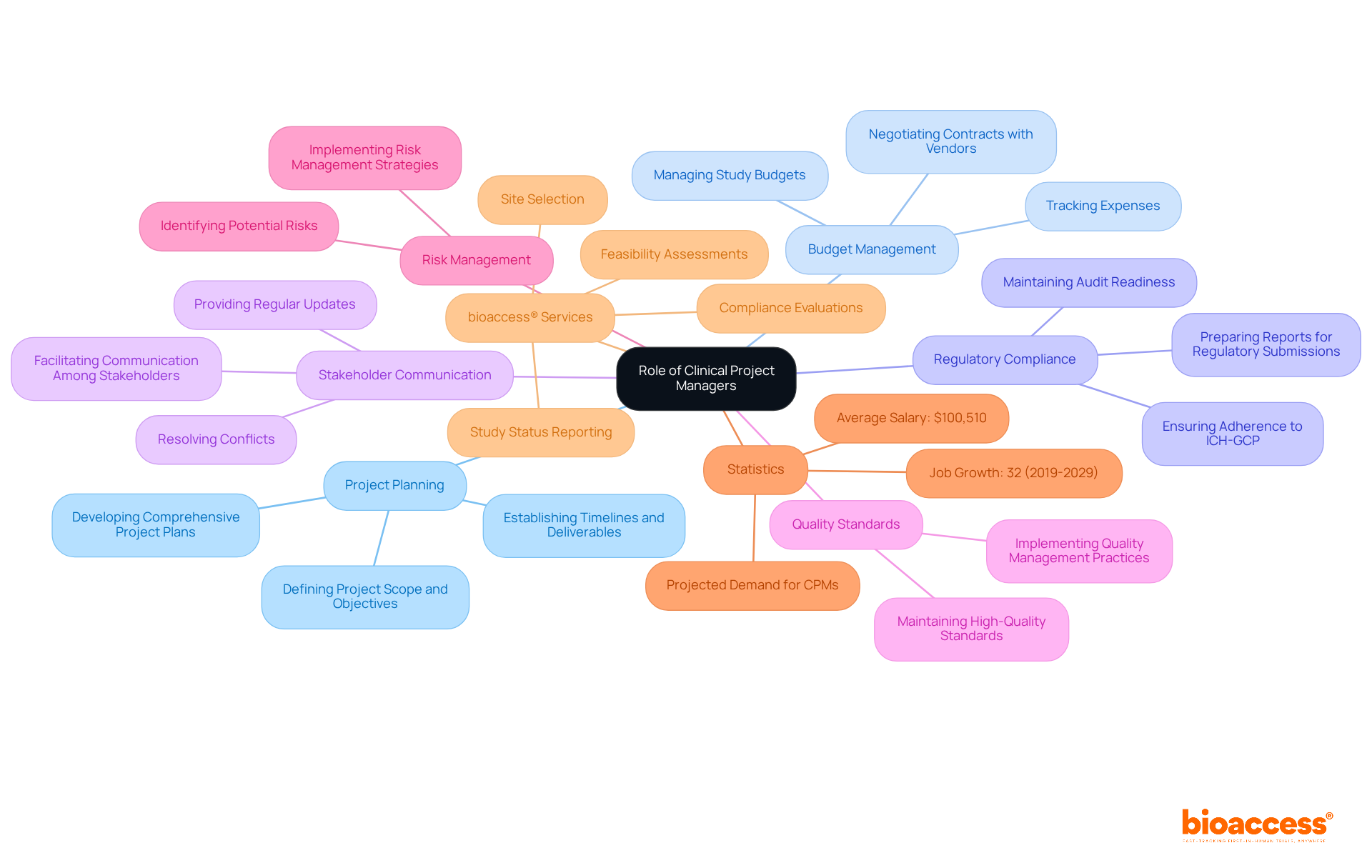
Successful clinical trials project management requires a blend of technical knowledge and interpersonal abilities. Key competencies include:
Project Coordination Expertise: Mastery of various coordination methodologies, such as Agile and Waterfall, is essential for effective planning and execution. Agile methodologies, for instance, have demonstrated a 64% higher success rate compared to traditional approaches, underscoring their effectiveness in dynamic environments.
Regulatory Knowledge: A solid understanding of Good Clinical Practice (GCP) and local regulatory requirements is crucial for ensuring compliance and minimizing risks throughout the study process.
Communication Skills: Effective communication is vital for coordinating with a diverse array of stakeholders, including sponsors, investigators, and regulatory bodies. Research indicates that documents are regarded as twice as significant as personal meetings in managing initiatives, emphasizing the necessity for clear written communication.
Leadership and Team Management: The capacity to guide and motivate a team is essential for sustaining morale and productivity during challenges. Successful project managers often oversee multiple projects simultaneously, necessitating strong leadership skills to navigate competing priorities.
Analytical Thinking: Strong analytical capabilities are essential for evaluating data, identifying trends, and making informed decisions based on trial outcomes. This skill set is becoming increasingly vital for clinical trials project management as the research environment evolves with new technologies and methodologies.
Problem-Solving Abilities: The ability to anticipate obstacles and devise effective solutions is essential in the fast-paced setting of medical research. With over half of initiatives failing to achieve their intended results, proactive problem-solving can significantly enhance outcomes.
By refining these abilities, healthcare managers can greatly improve their efficiency and contribute to the overall success of research studies.
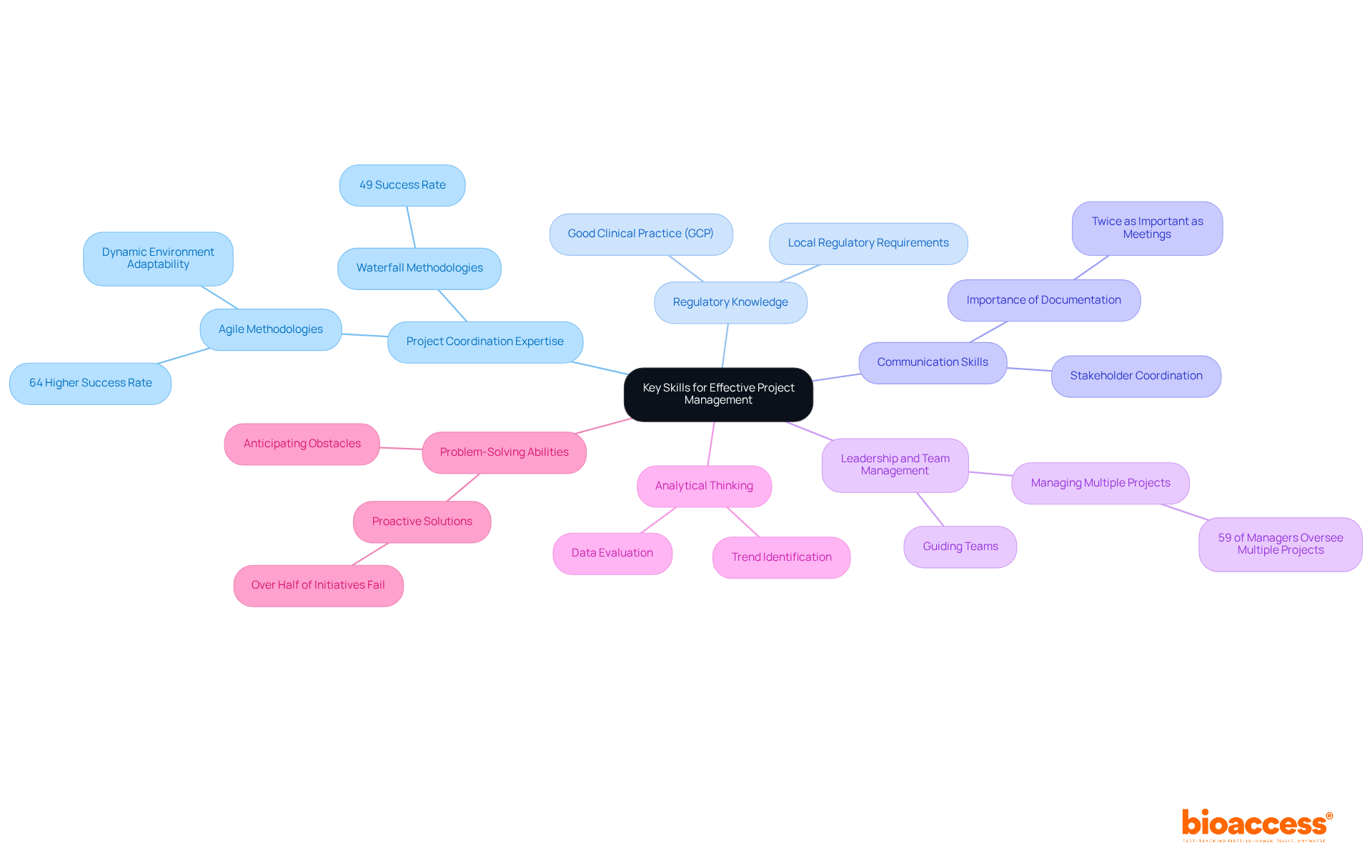
Project managers in the medical field encounter numerous obstacles that can hinder the progress of clinical trials project management. Among the most pressing issues are:
Recruitment Delays: Attracting suitable participants is a significant challenge, with nearly 90% of research studies struggling to meet their recruitment goals. To address this, Clinical Project Managers (CPMs) should implement comprehensive recruitment strategies for clinical trials project management that target diverse patient populations and leverage digital platforms. For example, utilizing mobile health applications and patient recruitment portals can enhance outreach and engagement, potentially boosting enrollment rates by as much as 50%.
Effective expense control is vital for success in clinical trials project management to avoid budget overruns. CPMs must develop detailed budgets and continuously monitor expenses, adjusting plans as needed to avoid overspending. Recruitment delays can lead to considerable financial losses, with estimates indicating that such delays can cost sponsors between $600,000 and $8 million per day.
Regulatory Compliance: Navigating the complex regulatory landscape is a challenge many CPMs encounter. Staying informed about regulatory changes and maintaining open communication with regulatory bodies is essential for clinical trials project management to ensure compliance and prevent costly delays.
Data Management Issues: Ensuring data integrity and security is critical in clinical studies. Implementing robust data management systems and conducting regular audits can help maintain high data quality, which is crucial for regulatory approval and the overall success of clinical trials project management.
Stakeholder Communication: Miscommunication among stakeholders can result in misunderstandings and delays in initiatives. Establishing clear communication protocols and providing regular updates can enhance collaboration and transparency, ensuring that all parties are aligned on objectives and timelines.
By proactively addressing these challenges, healthcare program managers can significantly improve the efficiency and effectiveness of clinical trials project management, ultimately accelerating the introduction of innovative therapies to the market.
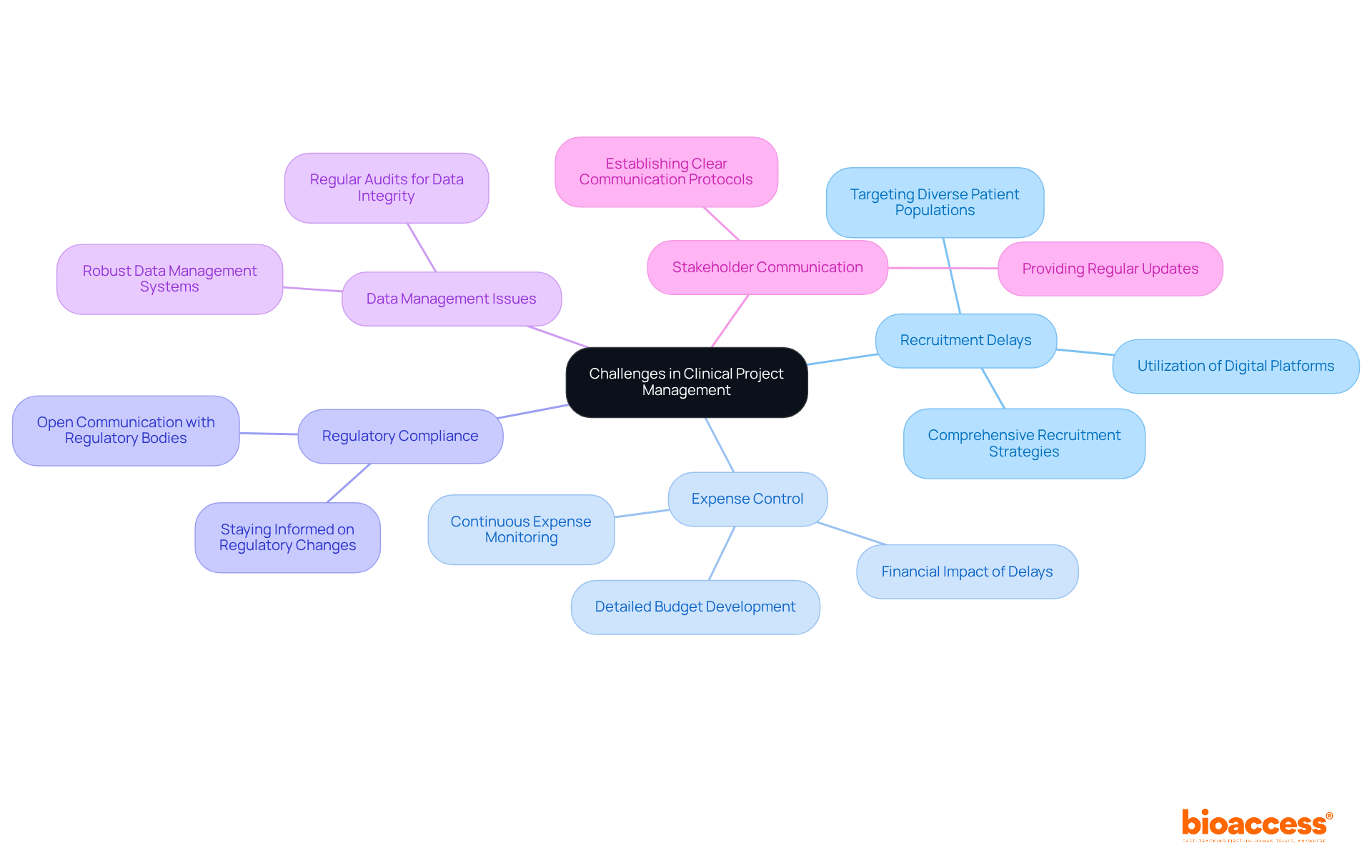
To improve healthcare program oversight, employing efficient methods and utilizing technology is essential. Key strategies include:
Adopting Agile Methodologies: The Agile approach promotes flexibility and adaptability in clinical trials. By dividing tasks into smaller, manageable phases, teams can swiftly respond to changes and challenges, enhancing overall efficiency. However, organizations may face challenges in adopting agile methodologies due to traditional top-down hierarchies, which can hinder collaboration and responsiveness.
Employing Clinical Trial Management Systems (CTMS): A CTMS centralizes data, tracks progress, and facilitates communication among team members, significantly improving project oversight efficiency. Research shows that organizations utilizing CTMS report a decrease in errors by as much as 30% and enhanced timelines, highlighting their importance in clinical studies. For instance, the "Budget Adherence Monitoring" case study illustrates how effective budget tracking can prevent cost overruns and ensure financial stability.
Incorporating Electronic Data Capture (EDC): EDC systems streamline data collection and management, ensuring real-time access to information and bolstering data integrity. This technology reduces the risk of data inconsistencies, which can disrupt study results. The 'Data Quality Metrics' case study emphasizes the significance of tracking errors and inconsistencies to uphold validity in the study.
Implementing Risk Control Frameworks: Creating a solid risk control framework enables early detection of possible risks and the formulation of efficient mitigation strategies, ensuring initiatives stay on course and within budget. Expert insights highlight the importance of incorporating risk management into clinical study workflows to improve overall success.
Leveraging Data Analytics: Employing data analytics tools offers essential insights into project performance, allowing project managers to make informed decisions and enhance processes. This data-oriented method fosters ongoing enhancement and increases the chances of successful outcomes. As highlighted by specialists, a thorough method to data examination can greatly enhance decision-making in medical studies.
Accelerating Patient Enrollment: By utilizing bioaccess®'s FDA-ready data, project managers can enroll treatment-naive cardiology or neurology cohorts 50% quicker than conventional Western sites, realizing substantial cost savings of $25K per patient. This capability tackles typical patient recruitment issues encountered by Medtech and biopharma startups, ensuring a more efficient study process.
Specialized Expertise in Medical Studies: bioaccess® focuses on managing various types of medical device research, including Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Medical Follow-Up Studies. This expertise guarantees that management of healthcare initiatives has the assistance necessary to navigate intricate study requirements efficiently.
By combining these methodologies and technologies, clinical project managers can greatly enhance their effectiveness and achieve successful results in clinical trials project management. The expected impact includes enhanced efficiency, reduced errors, and improved timelines, ultimately leading to more successful trial completions.
The success of clinical trials hinges significantly on the expertise and strategic approach of Clinical Project Managers (CPMs). These professionals are not only responsible for the meticulous planning and execution of trials but also play a vital role in ensuring compliance, managing budgets, and fostering communication among diverse stakeholders. Their multifaceted responsibilities underscore the importance of effective project management in advancing medical research and improving patient care.
Key insights regarding the essential skills required for CPMs have been shared, including:
Challenges such as:
have been highlighted, alongside strategies to mitigate these obstacles. The integration of methodologies like Agile and technologies such as Clinical Trial Management Systems (CTMS) further enhances the efficiency and effectiveness of clinical trials, paving the way for successful outcomes.
Ultimately, the role of clinical project managers is indispensable in navigating the complexities of clinical trials. By embracing best practices and leveraging innovative technologies, CPMs can significantly contribute to the acceleration of groundbreaking therapies reaching the market. Emphasizing the importance of continuous improvement and proactive problem-solving will not only enhance project outcomes but also reinforce the critical nature of effective project management in the evolving landscape of healthcare.
What is the role of Clinical Project Managers (CPMs) in clinical trials?
Clinical Project Managers play a crucial role in overseeing the entire process of clinical trials, from inception to completion. Their responsibilities include developing comprehensive project plans, managing budgets, ensuring compliance with regulatory standards, facilitating communication among stakeholders, balancing interests, maintaining quality standards, and implementing risk management strategies.
What are the key responsibilities of CPMs in clinical trials?
The key responsibilities of CPMs in clinical trials include developing project plans, managing budgets, ensuring regulatory compliance, facilitating communication among stakeholders, balancing interests of all parties, maintaining high-quality standards, and implementing risk management strategies.
How do CPMs ensure successful execution of clinical trials?
CPMs ensure successful execution of clinical trials by overseeing all aspects of the trial, implementing risk management strategies to identify potential issues, encouraging cooperation among cross-functional teams, and ensuring alignment with objectives and timelines.
What is the projected demand for project managers in healthcare?
The demand for project managers in healthcare is projected to grow significantly, driven by an aging population and advancing medical technologies.
What is the average salary of Clinical Project Managers?
The average salary of Clinical Project Managers is approximately $100,510, reflecting the substantial value of their contributions to medical research.
What services does the CPMs team at bioaccess® provide?
The CPMs team at bioaccess® provides extensive study coordination services, including feasibility assessments, site selection, compliance evaluations, setup, import permits, oversight, and reporting on study status and adverse events.
How does bioaccess® address challenges in the Latin American Medtech environment?
Bioaccess® addresses challenges in the Latin American Medtech environment by leveraging innovative solutions and efficient regulatory processes, which facilitate accelerated patient enrollment and regulatory approval, enabling faster access to groundbreaking medical technologies.