


Mastering close-out procedures under ALIMS guidelines is crucial for researchers navigating the intricate landscape of clinical compliance. By adhering to these standards, teams can not only sidestep costly pitfalls but also bolster the integrity and credibility of their studies. However, with regulations constantly evolving and the pressure to uphold ethical practices mounting, how can researchers ensure they are fully equipped for the challenges that lie ahead? This article explores best practices and strategic insights designed to empower research teams in effectively managing close-out procedures, ensuring compliance, and fostering trust in their findings.
The recommendations from the agency for medicines and medical devices are crucial for ensuring compliance with both local and international regulations in clinical studies. These protocols delineate the ethical and scientific criteria necessary for conducting clinical research in Serbia and other regions governed by the organization. A thorough understanding of these principles is vital for researchers to avoid common pitfalls that could lead to non-compliance, resulting in delays, financial penalties, or even the invalidation of study results.
Key elements of these standards include:
For instance, the streamlined ethics committee procedures in Serbia facilitate rapid approvals, often within 30 days, significantly enhancing the efficiency of clinical studies. This efficiency is further bolstered by the recent alignment of Serbia's regulatory framework with EU standards, which has made the approval process more transparent and effective.
As we approach 2025, anticipated revisions to ALIMS guidelines will further shape adherence procedures, necessitating that clinical research teams remain vigilant and adaptable. By familiarizing themselves with these evolving regulations, researchers can ensure their studies are not only compliant but also ethically sound, ultimately leading to better patient outcomes and more reliable data. The importance of adhering to ethical guidelines in clinical research cannot be overstated, as it fosters public trust and enhances the credibility of clinical studies.
At bioaccess®, we offer comprehensive clinical study management services, including:
Our expertise in navigating regulatory frameworks ensures that your clinical studies meet relevant standards, paving the way for a smoother path in advancing your medical device.
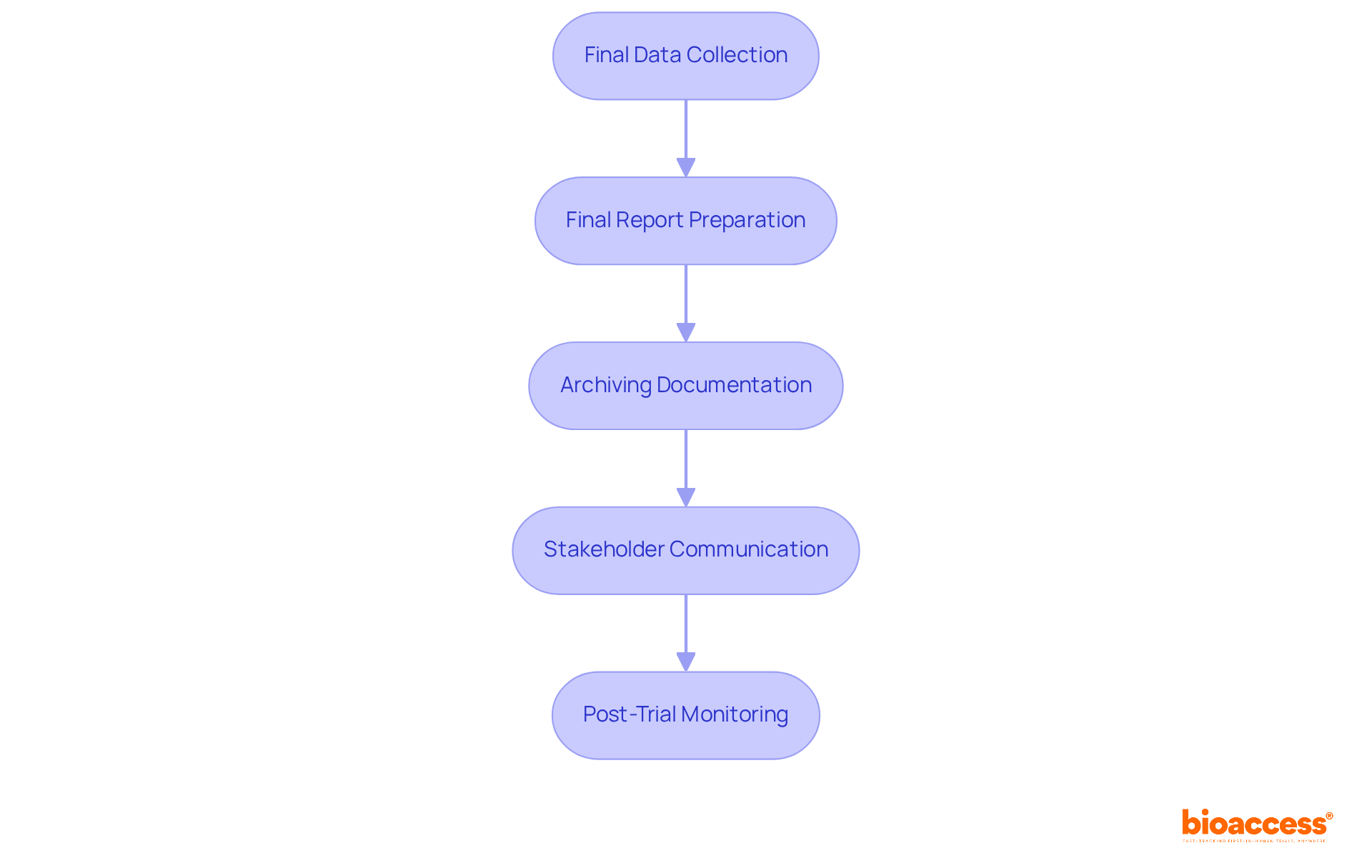
To effectively close out a clinical trial in accordance with ALIMS guidelines, researchers must adopt a structured approach that encompasses several essential steps:
Final Data Collection: Ensuring meticulous collection and verification of all data is crucial. This includes patient data, study results, and any adverse event reports, which are vital for thorough analysis.
Final Report Preparation: A detailed final report must be compiled, summarizing the trial's findings, methodologies, and any deviations from the original protocol. Submitting this report to ALIMS within the designated timeframe is essential for adherence.
Archiving Documentation: All trial-related documents, including consent forms, case report forms, and regulatory submissions, should be securely archived for future reference and audits. This practice not only aids in compliance but also supports transparency in research.
Stakeholder Communication: Informing all stakeholders, including sponsors and regulatory bodies, about the completion of the study is important. Supplying essential documentation ensures that all parties are aligned and informed of the results.
Post-Trial Monitoring: Establishing a strategy for observing any long-term effects on participants, if relevant, is crucial for ethical research practices and aids in comprehending the study's impact.
By following these steps, researchers can facilitate a seamless close-out procedure under ALIMS guidelines that meet necessary criteria while maintaining the integrity of the research conducted.
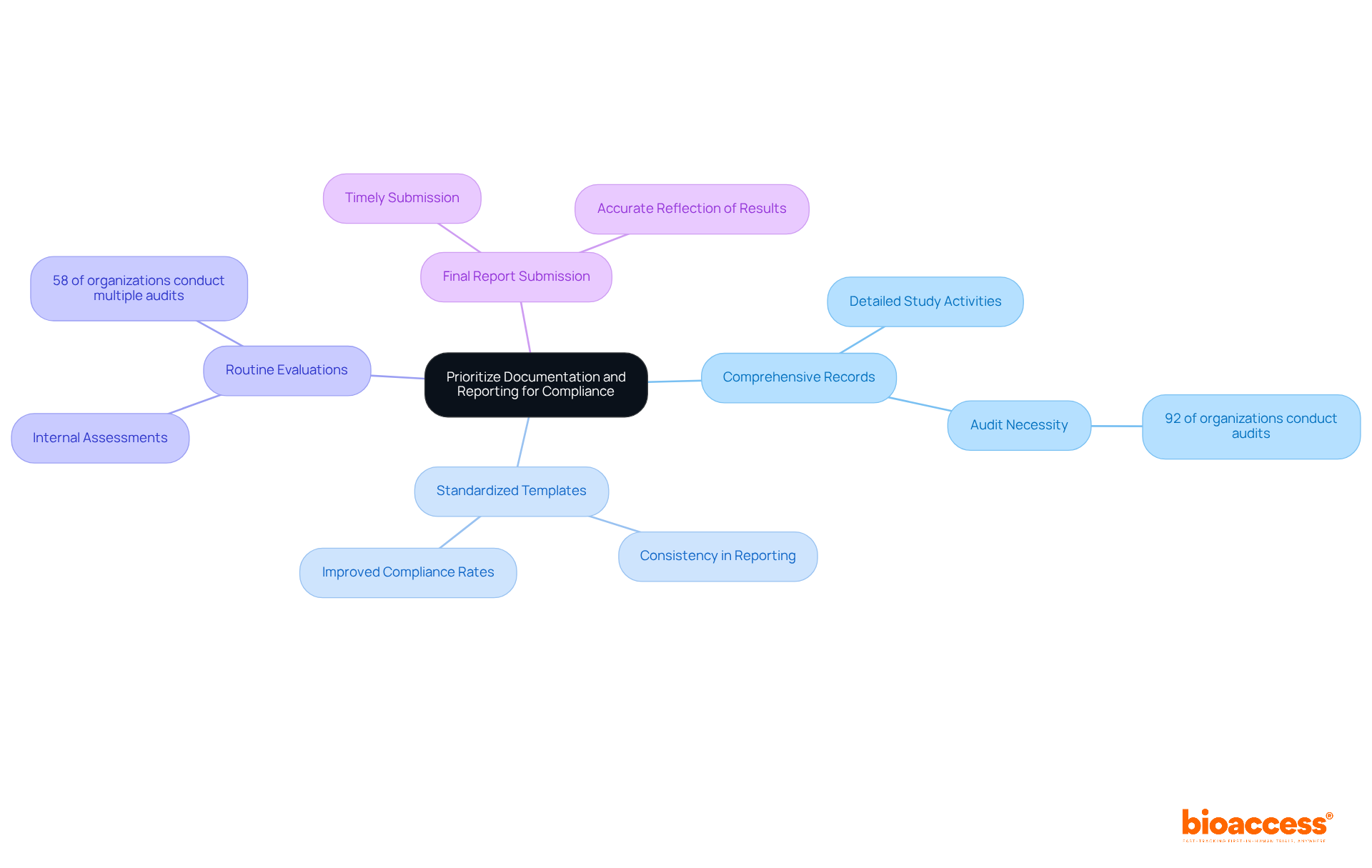
Effective documentation and reporting are crucial in clinical research, particularly for the close-out procedures under ALIMS guidelines. Researchers must adopt best practices to ensure compliance and efficiency:
Maintain Comprehensive Records: It's vital to keep detailed records of all study activities, including participant recruitment, consent processes, and data collection methods. This documentation should be organized and easily accessible for audits. In fact, 92% of organizations reported conducting at least two audits or assessments in 2025, underscoring the necessity of thorough record-keeping.
Use Standardized Templates: Implementing standardized templates for reports and documentation guarantees consistency and completeness. This practice streamlines the reporting process and reduces the risk of omitting critical information. Case studies show that organizations using standardized templates see improved compliance rates and better audit outcomes.
Routine Evaluations: Conducting internal assessments throughout the process is essential to ensure that documentation remains current and adheres to guidelines. This proactive approach helps identify potential issues before the final close-out, as 58% of organizations reported conducting four or more audits in 2025.
Final Report Submission: It's imperative to submit the final report to the designated system within the required timeframe, including all necessary appendices and supporting documents. This report should accurately reflect the results of the experiment and any deviations from the original protocol. Effective reporting is linked to improved adherence and smoother close-out processes.
By prioritizing documentation and reporting, researchers can significantly enhance adherence and facilitate a more effective trial conclusion through close-out procedures under ALIMS guidelines.
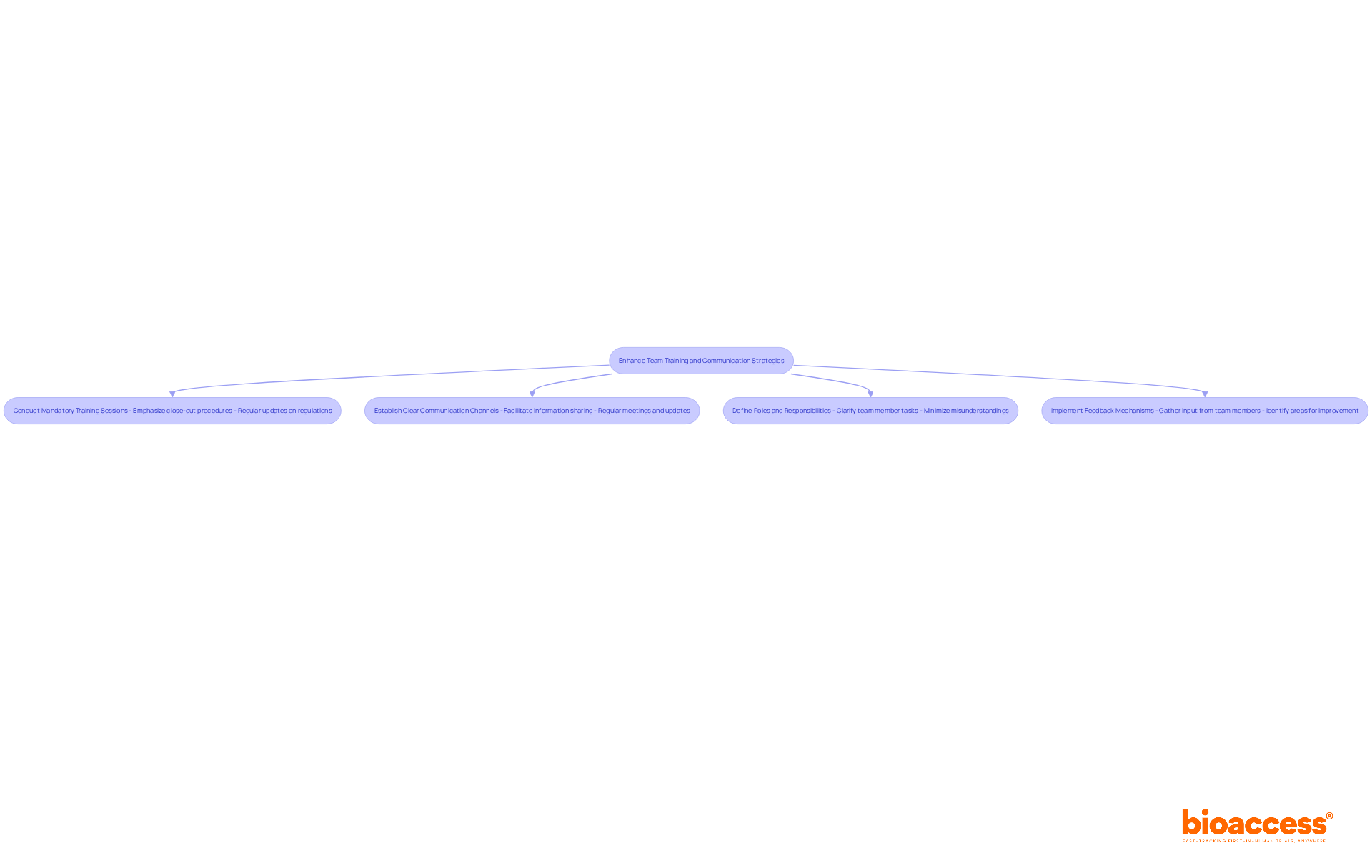
To ensure compliance with established standards during the close-out process, enhancing team training and communication strategies is crucial. Here are some best practices to consider:
Conduct mandatory training sessions that emphasize close-out procedures under ALIMS guidelines and other ALIMS guidelines. These sessions should be updated regularly to reflect any changes in regulations, ensuring that all team members remain informed and compliant.
Clear Communication Channels: Establish clear communication channels within the team to facilitate the sharing of information and updates regarding the close-out process. Regular meetings, email updates, and collaborative platforms can enhance transparency and keep everyone aligned.
Role Clarity: Clearly define the roles and responsibilities of each team member involved in the close-out process. This clarity helps avoid misunderstandings and ensures that all tasks are accomplished efficiently, minimizing the risk of regulatory issues.
Feedback Mechanisms: Implement feedback mechanisms to gather input from team members on the close-out process. This feedback is invaluable for identifying areas for improvement and enhancing overall adherence. Research indicates that effective communication strategies can significantly boost adherence rates, with organizations reporting up to a 25% increase in protocol compliance when feedback is actively sought and implemented.
By enhancing training and communication strategies, research teams can cultivate a culture of compliance, ensuring that all members are well-prepared for the close-out procedures under ALIMS guidelines while effectively meeting regulatory standards.
Mastering close-out procedures under ALIMS guidelines is not just important; it’s essential for ensuring compliance and maintaining the integrity of clinical research. By grasping and implementing these guidelines, researchers can effectively navigate the complexities of clinical trials. This ultimately leads to improved patient outcomes and enhances credibility in the field. Adhering to ALIMS standards mitigates risks associated with non-compliance and fosters public trust in clinical studies.
Key insights from the article underscore the significance of:
Researchers must prioritize:
Continuous team training and the use of standardized templates can significantly enhance compliance and streamline the close-out process, ensuring that all regulatory requirements are met.
As the landscape of clinical research evolves, staying informed about ALIMS guidelines and best practices becomes increasingly vital. Researchers are urged to embrace these practices and cultivate a culture of compliance within their teams. By doing so, they can navigate the challenges of ALIMS close-out procedures and contribute to the advancement of medical science and the betterment of patient care.
What are ALIMS guidelines and why are they important?
ALIMS guidelines are recommendations from the agency for medicines and medical devices that ensure compliance with local and international regulations in clinical studies. They delineate the ethical and scientific criteria necessary for conducting clinical research, helping researchers avoid non-compliance issues that could lead to delays, financial penalties, or invalidation of study results.
What are the key elements of the ALIMS guidelines?
Key elements of the ALIMS guidelines include requirements for informed consent, data integrity, and a clear definition of responsibilities for sponsors and researchers.
How do the ethics committee procedures in Serbia benefit clinical studies?
The streamlined ethics committee procedures in Serbia facilitate rapid approvals, often within 30 days, which significantly enhances the efficiency of clinical studies.
How has Serbia's regulatory framework changed recently?
Serbia's regulatory framework has recently aligned with EU standards, making the approval process for clinical studies more transparent and effective.
What future changes to ALIMS guidelines are anticipated?
Anticipated revisions to ALIMS guidelines as we approach 2025 will shape adherence procedures, requiring clinical research teams to remain vigilant and adaptable to ensure compliance.
Why is adherence to ethical guidelines in clinical research important?
Adhering to ethical guidelines is crucial as it fosters public trust and enhances the credibility of clinical studies, ultimately leading to better patient outcomes and more reliable data.
What services does bioaccess® offer for clinical study management?
Bioaccess® offers comprehensive clinical study management services, including feasibility studies, site selection, compliance reviews, study setup, import permits, project management, and reporting on study status.
Recommendations for Clinical Trials Ending Early | AAIC | alz.org (https://aaic.alz.org/releases_2022/participant-first.asp)
30 Quotes About the Future of Healthcare: Expert Takes (https://deliberatedirections.com/quotes-future-of-healthcare)
Time to Publication of Clinical Trials Funded by The NINDS - PMC (https://pmc.ncbi.nlm.nih.gov/articles/PMC8679128)
Best Practices
Case Studies (https://ors.od.nih.gov/OD/OQM/benchmarking/bestpractice/Pages/case_studies.aspx)