


The article focuses on best practices for mastering design verification in the medical device industry to ensure clinical success. It emphasizes the importance of structured design validation processes, regulatory compliance, and effective documentation and communication strategies. These elements are essential for reducing risks and enhancing product reliability and safety. In the ever-evolving Medtech landscape, understanding these best practices is crucial for addressing the key challenges faced by professionals in clinical research. Collaboration among stakeholders is vital to navigate these complexities and drive innovation in product development.
Mastering design verification is a critical component in the journey to successful clinical outcomes for medical devices. As the industry faces increasing regulatory scrutiny and demands for safety and efficacy, understanding and implementing best practices in design verification becomes essential. Organizations must ensure that their verification processes not only meet regulatory standards but also lead to innovative and reliable products. This article delves into the fundamental principles of design verification, exploring strategies that enhance quality assurance while navigating the complexities of compliance in the medical device landscape.
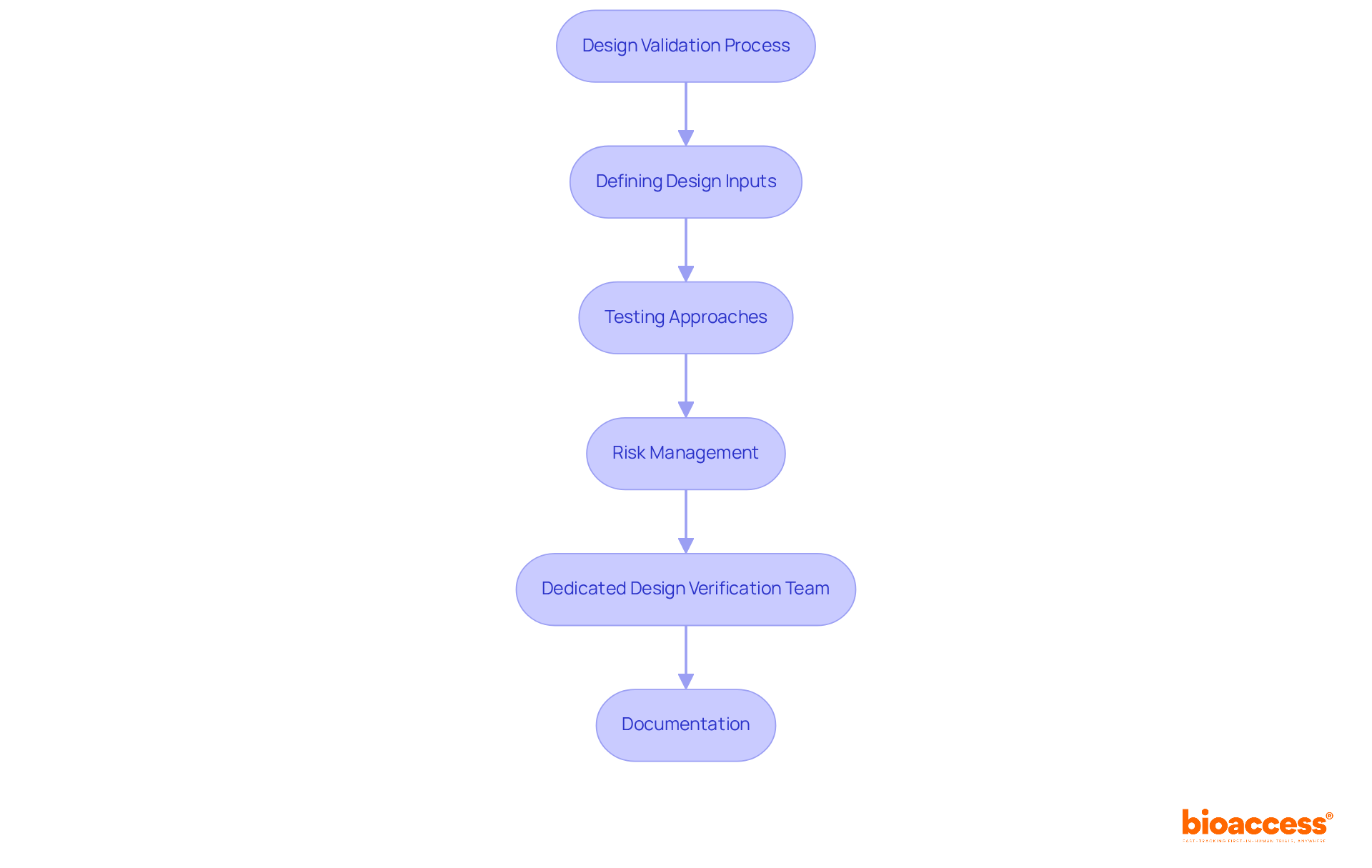
Design validation is a structured method that ensures a medical instrument fulfills its criteria—specifications and requirements set at the start of development. This process is crucial for guaranteeing that the apparatus operates as intended under specified conditions. Key components of design validation include:
Creating a strong assessment method is essential for reducing risks and improving the chances of clinical success. By prioritizing these elements, organizations can navigate the complexities of medical equipment development more effectively, ultimately leading to safer and more efficient products.
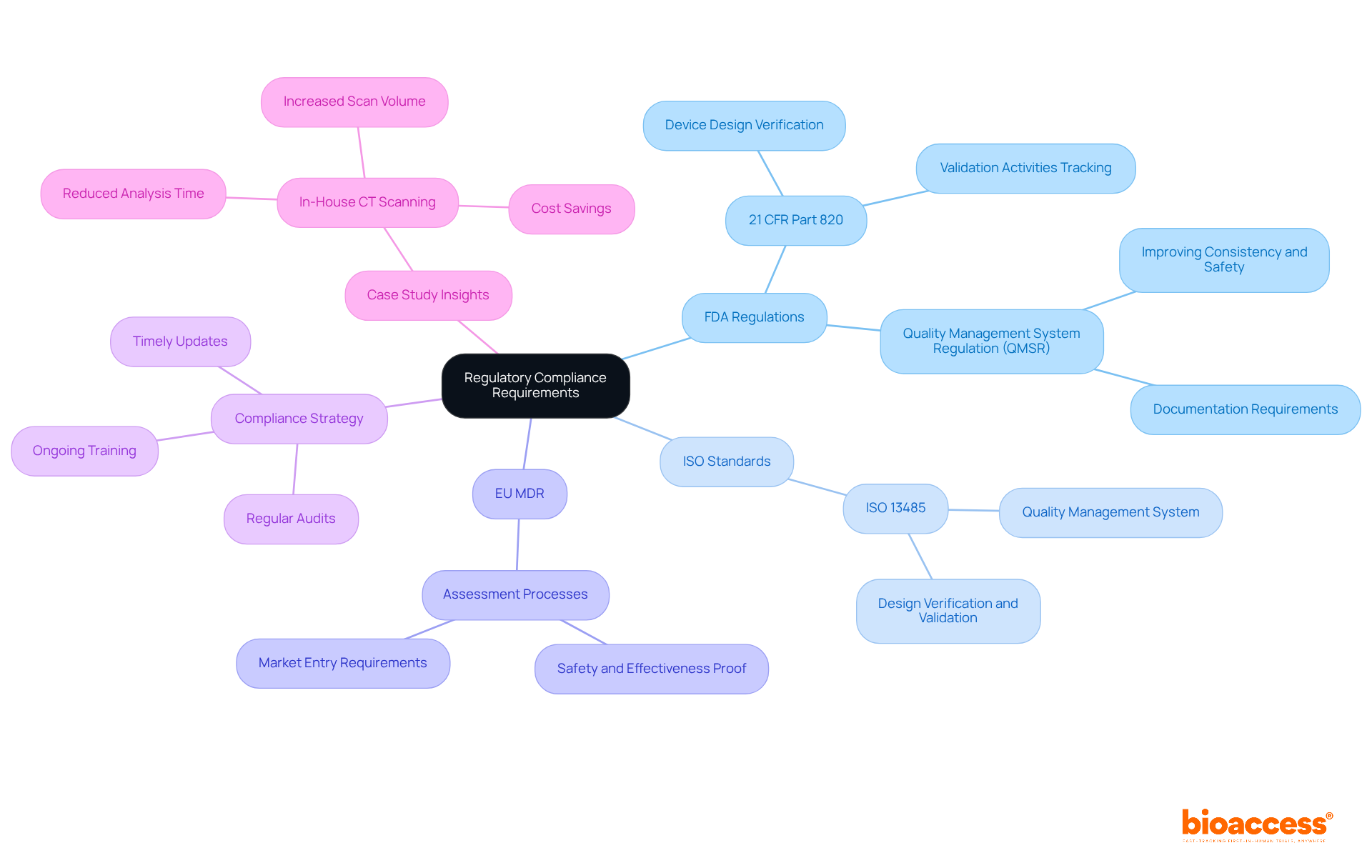
Regulatory adherence is paramount in the assessment process for medical equipment, ensuring that products meet stringent safety and effectiveness criteria. Key regulations include:
FDA Regulations: Under 21 CFR Part 820, manufacturers must establish and maintain procedures that verify device designs against specified requirements. This mandates comprehensive assessment reviews and meticulous tracking of all validation activities.
ISO Standards: Compliance with ISO 13485 is essential for organizations, as it guarantees the establishment of a quality management system that aligns with global standards for medical equipment. This standard encompasses specific criteria for design verification and validation methods, which are critical for maintaining product integrity.
EU MDR: The European Union Medical Equipment Regulation enforces rigorous assessment and validation processes, necessitating that products are proven safe and effective prior to market entry.
Organizations should implement a comprehensive compliance strategy that integrates regular audits, ongoing training, and timely updates to ensure adherence to these regulations throughout the product lifecycle. A recent case study exemplified how a multinational medical technology company enhanced its compliance by incorporating in-house CT scanning solutions, significantly reducing analysis time from three weeks to just a few minutes while meeting FDA requirements. This proactive approach not only streamlines operations but also reinforces the organization’s commitment to quality and safety in medical product development.
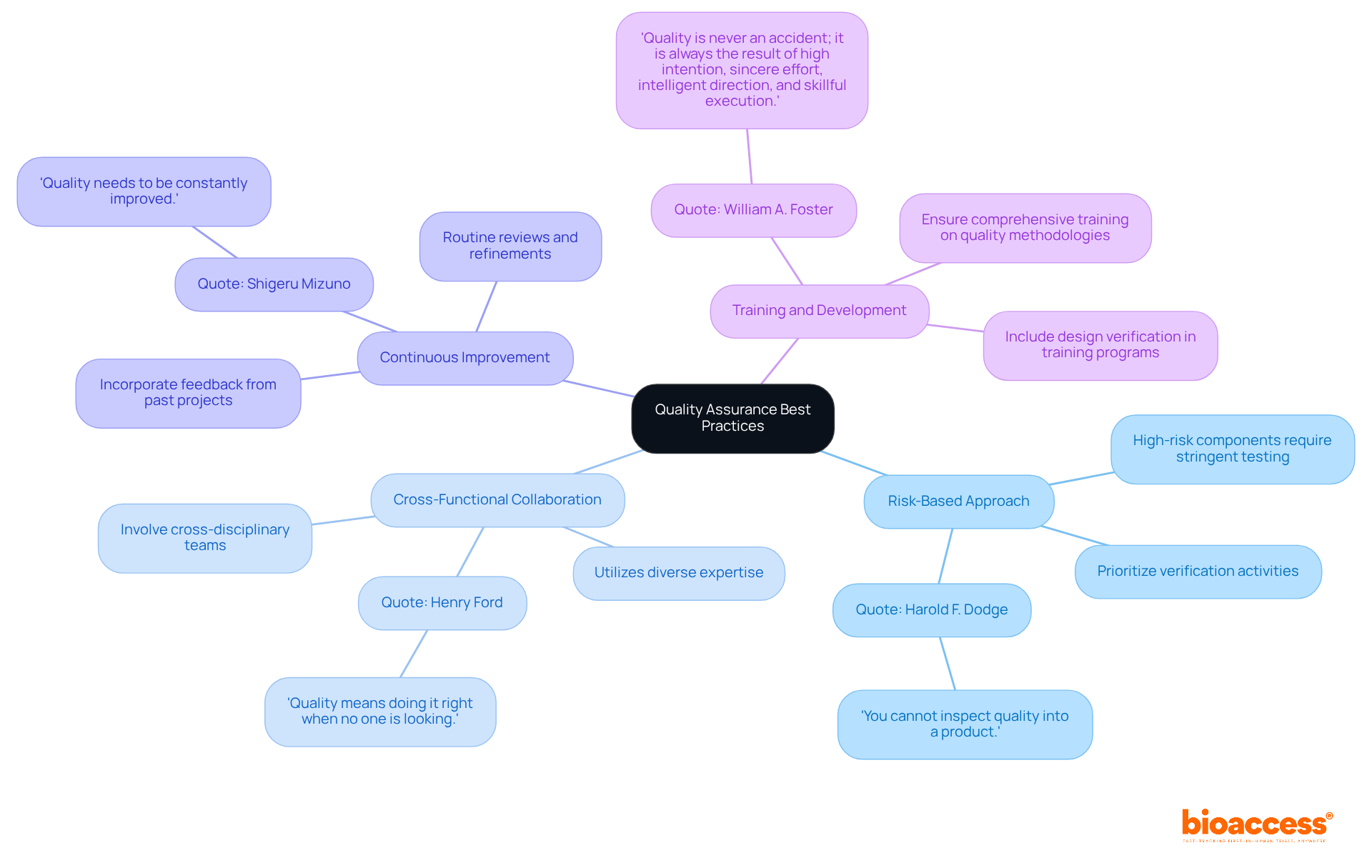
To achieve superior quality outcomes in design verification, organizations must implement the following best practices:
By embracing these best practices, organizations can bolster their quality assurance efforts, ultimately resulting in increased reliability of equipment and enhanced patient safety. However, it is crucial to be aware of common pitfalls, such as neglecting to involve all relevant stakeholders or failing to adapt processes based on feedback, which can hinder the effectiveness of these practices.
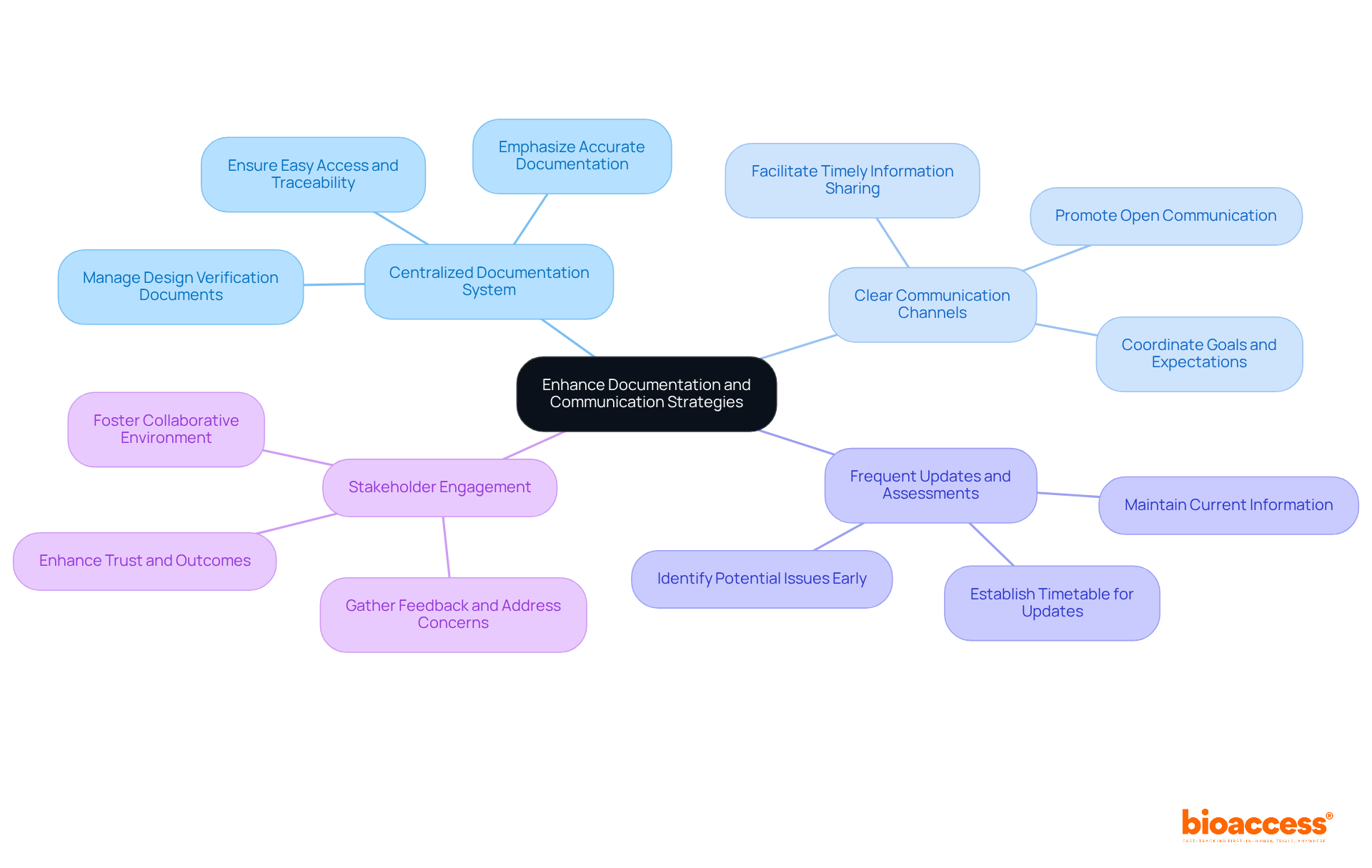
Improving documentation and communication approaches is crucial for successful assessment in the medical device industry. Key recommendations include:
By prioritizing effective documentation and communication, organizations can significantly enhance their design verification processes, which results in improved outcomes and stronger regulatory compliance.
Mastering design verification is not just beneficial; it is essential for ensuring the clinical success of medical devices. By adhering to best practices in design validation, organizations can significantly enhance the reliability and safety of their products. A structured approach to defining design inputs, implementing rigorous testing, and maintaining thorough documentation lays the groundwork for effective design verification processes.
Key insights from this article underscore the importance of:
Employing a risk-based approach, fostering continuous improvement, and engaging stakeholders throughout the design verification process are crucial steps in navigating the complexities of medical device development. These strategies not only streamline operations but also reinforce a commitment to quality and safety.
Ultimately, organizations are urged to prioritize design verification as a fundamental component of their product development strategy. By doing so, they can improve clinical outcomes, enhance patient safety, and ensure compliance with regulatory standards. Embracing these practices will not only lead to successful product launches but also contribute to a culture of excellence within the medical device industry.
What is design validation in the context of medical instruments?
Design validation is a structured method that ensures a medical instrument meets its specified criteria and requirements set at the start of development, confirming that the device operates as intended under specified conditions.
What are the key components of design validation?
The key components include defining design inputs, utilizing various testing approaches, implementing risk management, establishing a dedicated design verification team, and maintaining comprehensive documentation of all validation activities.
Why is defining design inputs important?
Defining design inputs is crucial as it clearly articulates the requirements the device must fulfill, including performance, safety, and usability criteria, which sets the foundation for all subsequent design verification activities.
What testing approaches are used in design validation?
Various testing approaches include inspections, analyses, and performance tests to confirm that outputs correspond with specified inputs, helping to identify potential issues early in the development cycle.
How does risk management contribute to design validation?
A strong risk management approach helps recognize and reduce possible dangers early in the development process, enhancing product reliability and safety, and ensuring compliance with regulatory standards.
What is the role of a dedicated design verification team?
A dedicated design verification team provides a fresh perspective, identifies potential issues early in the development cycle, and helps maintain a broader view of user and regulatory requirements, aiding the success of the validation procedure.
Why is documentation important in the design validation process?
Maintaining comprehensive records of all validation activities provides evidence of compliance and facilitates regulatory review, ensuring that all necessary information is readily available for assessment.
How can organizations improve the chances of clinical success in medical equipment development?
By prioritizing key elements such as risk management, testing, and documentation, organizations can reduce risks and navigate the complexities of medical equipment development more effectively, leading to safer and more efficient products.