


Navigating the complex landscape of drug dossier submission to HALMED can be daunting, particularly with the constantly changing regulatory frameworks that govern medicinal products. It's crucial to grasp the nuances of the Medicinal Products Act and the European Medicines Agency guidelines to ensure compliance and boost the chances of approval. But with so much at stake, what happens when applicants face common pitfalls that could derail their submissions? This guide provides a comprehensive, step-by-step approach to mastering the drug dossier submission process, equipping you with the knowledge and strategies necessary to overcome challenges and achieve successful outcomes.
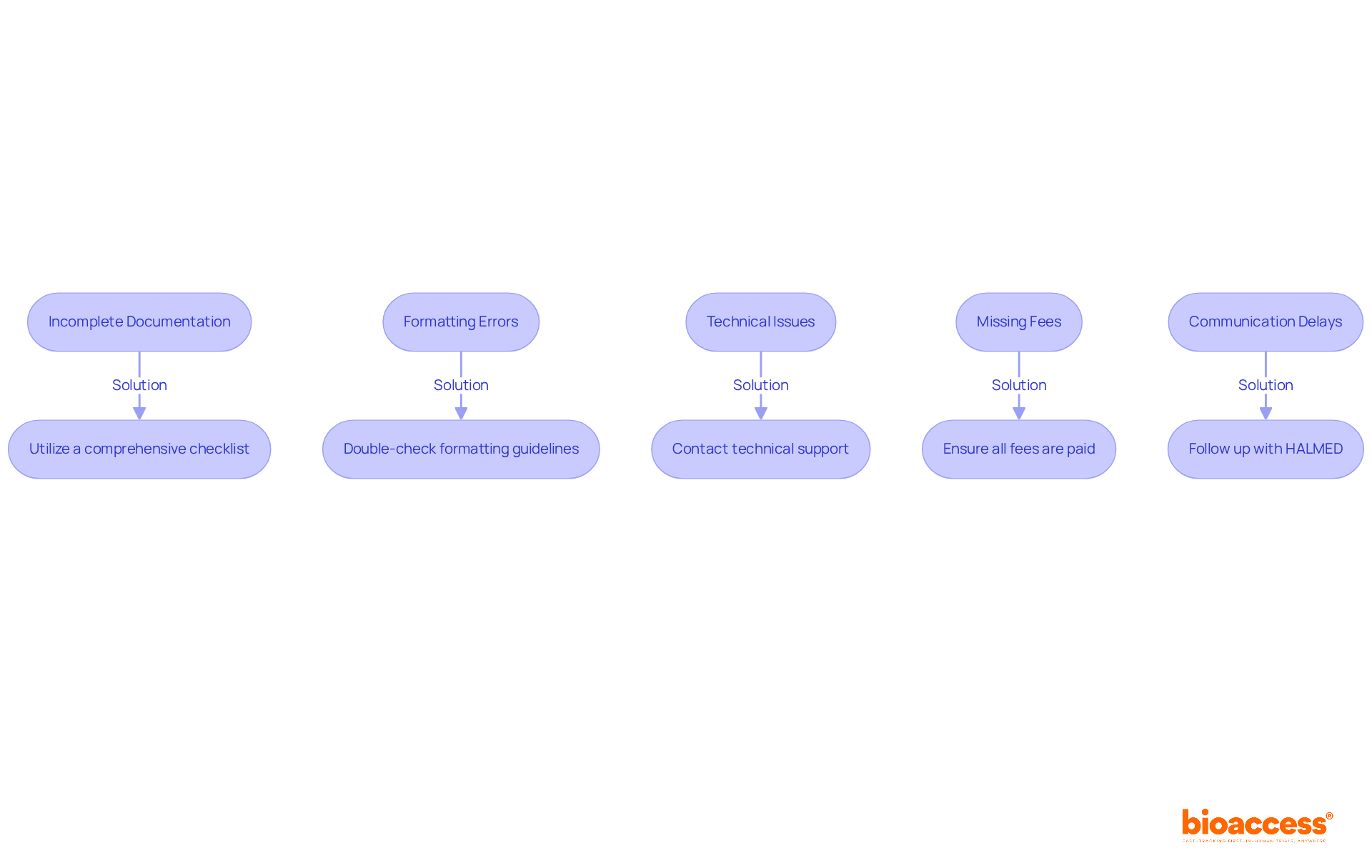
Before embarking on the drug dossier submission to HALMED, it’s crucial to grasp the Medicinal Products Act and the relevant European Union regulations. This foundational knowledge includes understanding the roles of various regulatory agencies and the specific criteria for pharmaceutical applications in Croatia. Key documents to review are:
Understanding these regulations not only aligns your submission with legal expectations but also significantly enhances the likelihood of approval. For instance, successful drug dossier submission to Halmed has demonstrated that adherence to these guidelines can lead to expedited approval timelines, markedly shorter than in many other regions. In fact, the average medication approval timeline in Croatia has improved, reflecting the efficacy of these regulatory frameworks. By leveraging the aligned standards set forth by the EMA and other relevant bodies, you can refine your proposal strategy and facilitate the successful launch of innovative therapies into the market.
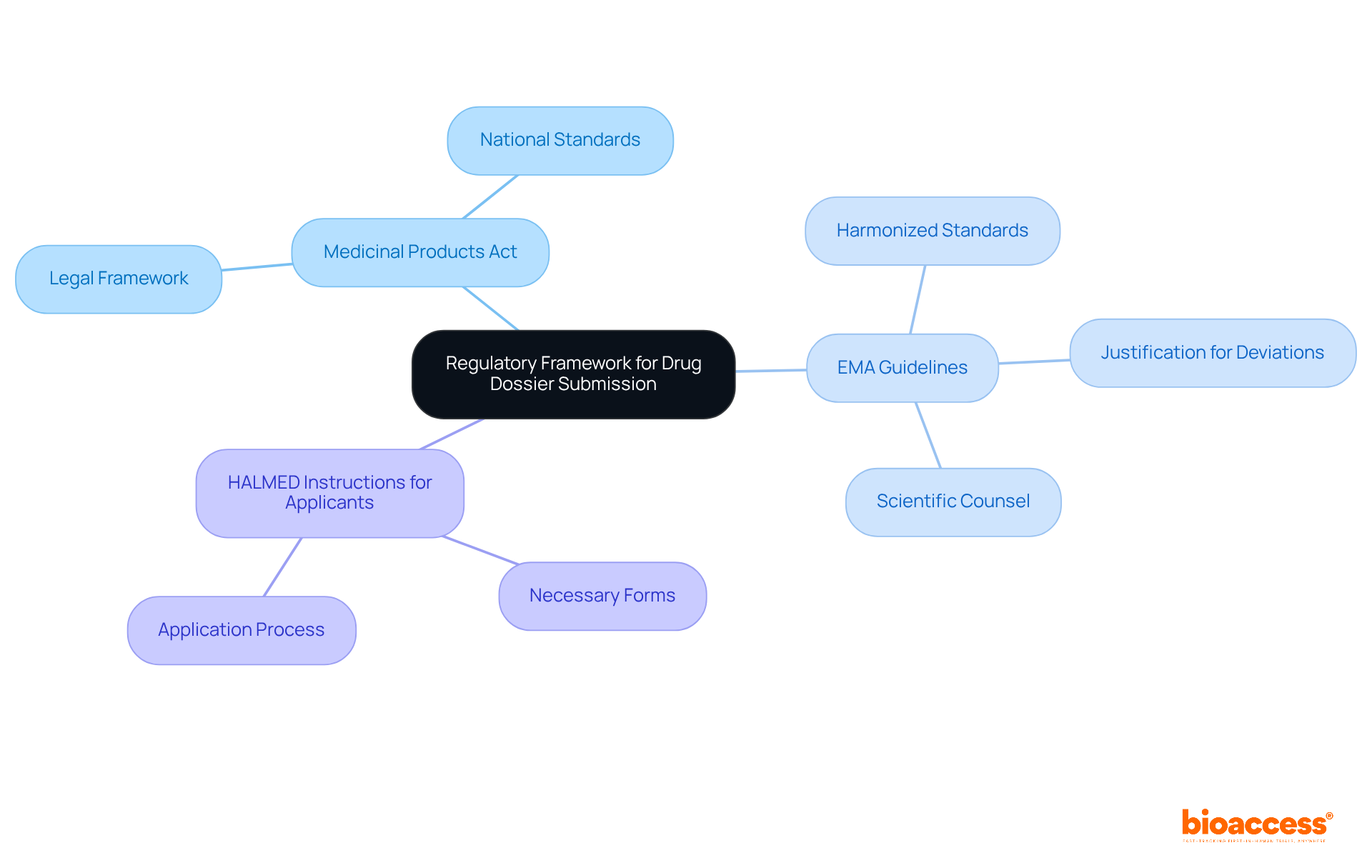
To prepare your drug dossier for submission to HALMED, it is essential to gather the following key documents:
It is important to ensure that all documents are complete, accurate, and formatted correctly for the drug dossier submission to HALMED. Frequent documentation mistakes can result in considerable delays in the approval procedure, so careful preparation is essential. Interacting with regulatory specialists can further simplify the filing process, enhancing compliance and facilitating a smoother review.
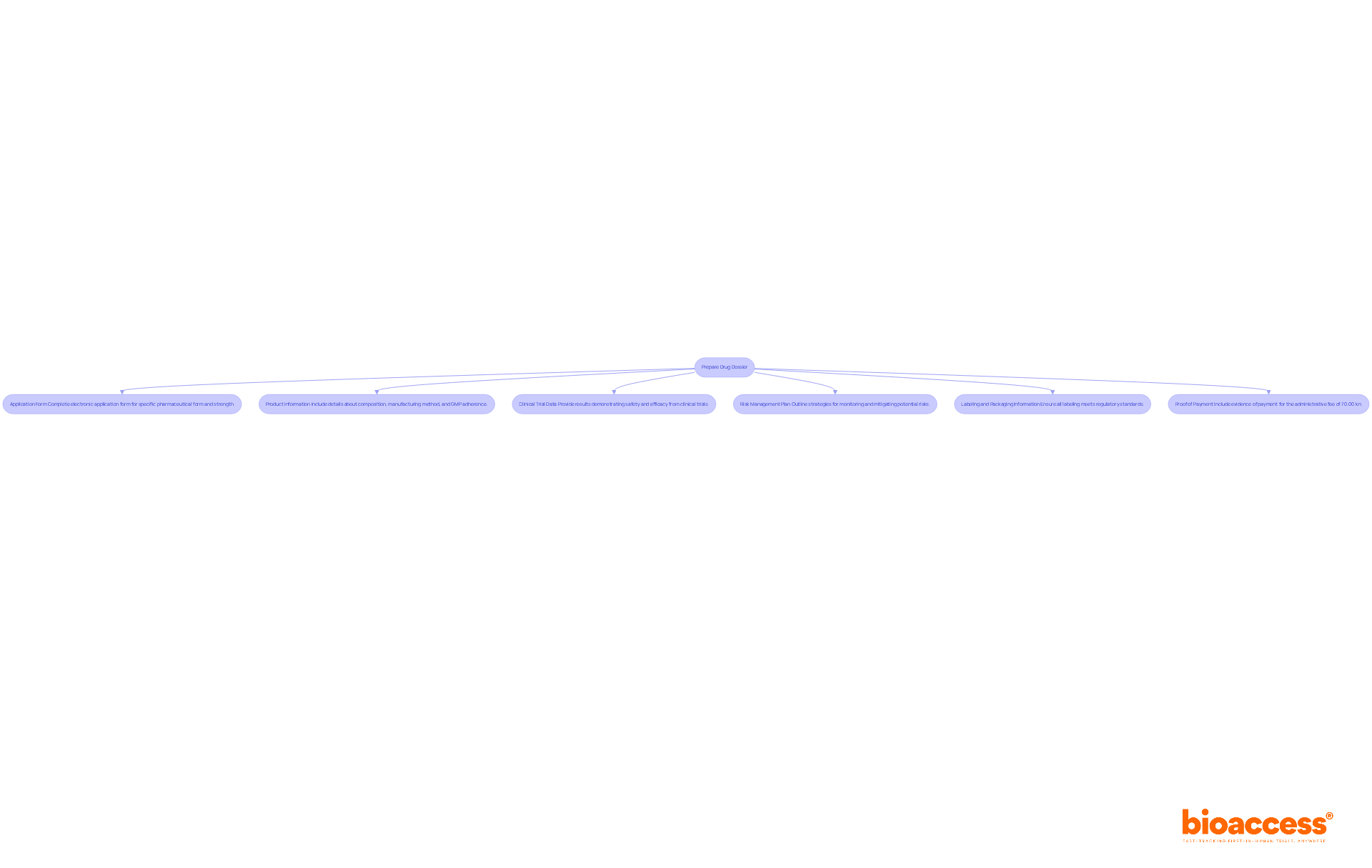
To successfully submit your drug dossier to HALMED, follow these essential steps:
Additionally, note that proof of payment is a mandatory attachment to the electronic Application Form (eAF) for variation procedures. With the Pharmaceuticals market in Croatia projected to reach US$426.97 million by 2025, ensuring a successful application can significantly impact your market entry strategy. Integrating optimal methods from regulatory affairs experts can further enhance your filing approach. By adhering to these steps, you can improve the success rate of your drug dossier submission to HALMED, ensuring a smoother and more efficient procedure.
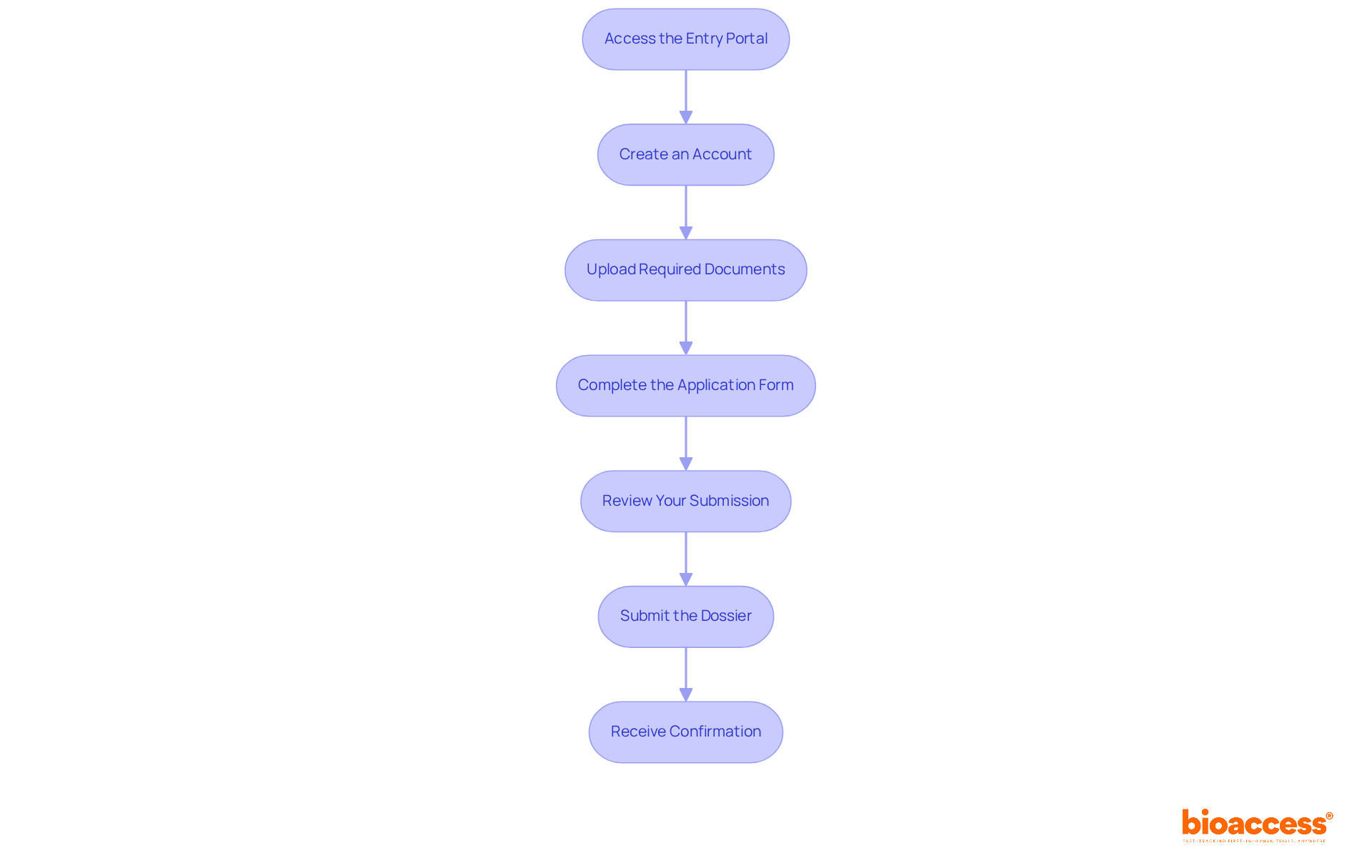
During the drug dossier submission to halmed process, applicants often encounter several typical challenges that can obstruct approval. Understanding these hurdles is crucial for anyone involved in clinical research. Here are essential troubleshooting tips to navigate these issues effectively:
Incomplete Documentation: A significant percentage of application rejections stem from incomplete documentation. To mitigate this risk, utilize a comprehensive checklist to ensure all necessary documents are included prior to sending.
Formatting Errors: Following the specific formatting guidelines set by the organization is crucial. Poorly formatted entries can lead to rejections or unnecessary delays, so double-check that your documents meet all outlined requirements.
Technical Issues: If you face technical difficulties with the filing portal, quickly contact the organization's technical support for help. Timely communication can prevent prolonged setbacks.
Missing Fees: Ensure that all applicable fees are paid in full. Missing payments can interrupt the evaluation, leading to additional delays in your timeline.
Communication Delays: If you do not receive confirmation of your entry within a reasonable timeframe, proactively follow up with HALMED. This step is vital to confirm that your application was received and is being processed.
By proactively addressing these common issues, you can significantly enhance the efficiency of your submission process and improve the likelihood of obtaining timely approval. Consider how these strategies can be integrated into your own practices to streamline your clinical research efforts.
Mastering the drug dossier submission process to HALMED is crucial for any pharmaceutical entity looking to introduce new medications to the Croatian market. This guide highlights the necessity of understanding the regulatory framework, preparing comprehensive documentation, and effectively navigating the submission process to boost approval chances.
Key insights emphasize the importance of adhering to the Medicinal Products Act and EMA guidelines. Compiling essential documents, such as:
is vital, along with following a structured submission process. Moreover, addressing common issues like incomplete documentation and formatting errors can significantly streamline the application journey.
Ultimately, successful drug dossier submissions not only facilitate market entry but also enhance the overall efficiency of the pharmaceutical landscape in Croatia. By implementing the strategies outlined in this guide, stakeholders can adeptly navigate the complexities of the regulatory environment, ensuring that innovative therapies reach patients promptly. Embracing these best practices is essential for gaining a competitive edge in the rapidly growing Croatian pharmaceutical market, projected to experience substantial growth by 2025.
What is the importance of understanding the regulatory framework for drug dossier submission to HALMED?
Understanding the regulatory framework is crucial for aligning submissions with legal expectations and enhancing the likelihood of approval.
What key documents should be reviewed before submitting a drug dossier to HALMED?
Key documents include the Medicinal Products Act, European Medicines Agency (EMA) Guidelines, and HALMED Instructions for Applicants.
What does the Medicinal Products Act establish?
The Medicinal Products Act establishes the legal framework for drug approval in Croatia, ensuring adherence to national standards.
What do the EMA Guidelines provide for drug submissions?
The EMA Guidelines offer harmonized standards across EU member states and promote a consistent approach to drug submissions, requiring applicants to justify any deviations.
What are HALMED Instructions for Applicants?
HALMED Instructions for Applicants detail the necessary forms and paperwork required for drug dossier submission to HALMED, streamlining the application process.
How can adherence to these regulatory guidelines impact the approval timeline?
Adherence to these guidelines can lead to expedited approval timelines, which are significantly shorter than in many other regions.
What has been the impact of these regulatory frameworks on medication approval timelines in Croatia?
The average medication approval timeline in Croatia has improved, reflecting the efficacy of the regulatory frameworks established by the EMA and other relevant bodies.