Effective management of drug import and distribution tracking is essential in Croatian clinical trials, where regulatory compliance and patient safety take precedence. This guide explores the critical components of establishing a robust tracking system, empowering stakeholders to ensure that investigational medicinal products are managed with integrity and efficiency. Given the complex regulatory landscape and the rapid evolution of technology, how can stakeholders effectively navigate the challenges of maintaining compliance while optimizing the tracking process?
By addressing these questions, we can better understand the Medtech landscape and the pivotal role that bioaccess plays in overcoming key challenges. Collaboration among stakeholders is vital to streamline processes and enhance patient safety. As we delve deeper into this topic, we will uncover strategies that not only meet regulatory demands but also foster innovation in clinical research.
To effectively manage drug import and distribution tracking in Croatian clinical trials, it’s essential to grasp several key concepts:
Drug Importation: This is the legal process of bringing investigational medicinal products (IMPs) into Croatia. Familiarize yourself with the necessary documentation, including import licenses and compliance with local regulations.
Drug import and distribution tracking in Croatian trials involves the systematic monitoring of medication movement from the point of importation to the clinical trial sites. It ensures that medications are delivered safely and efficiently, maintaining their integrity throughout the process.
Regulatory Compliance: Understanding the Croatian regulations governing medicine importation and distribution is vital. This includes adherence to the Act on Medicinal Products and any specific guidelines set forth by HALMED, the Croatian Agency for Medicinal Products and Medical Devices.
Technology Utilization: Acquaint yourself with contemporary monitoring technologies, such as RFID and IoT, which can enhance the efficiency of medication oversight and ensure real-time visibility of item status throughout the supply chain.
By mastering these fundamentals, you will be better equipped to implement effective tracking systems in your clinical studies.
![]()
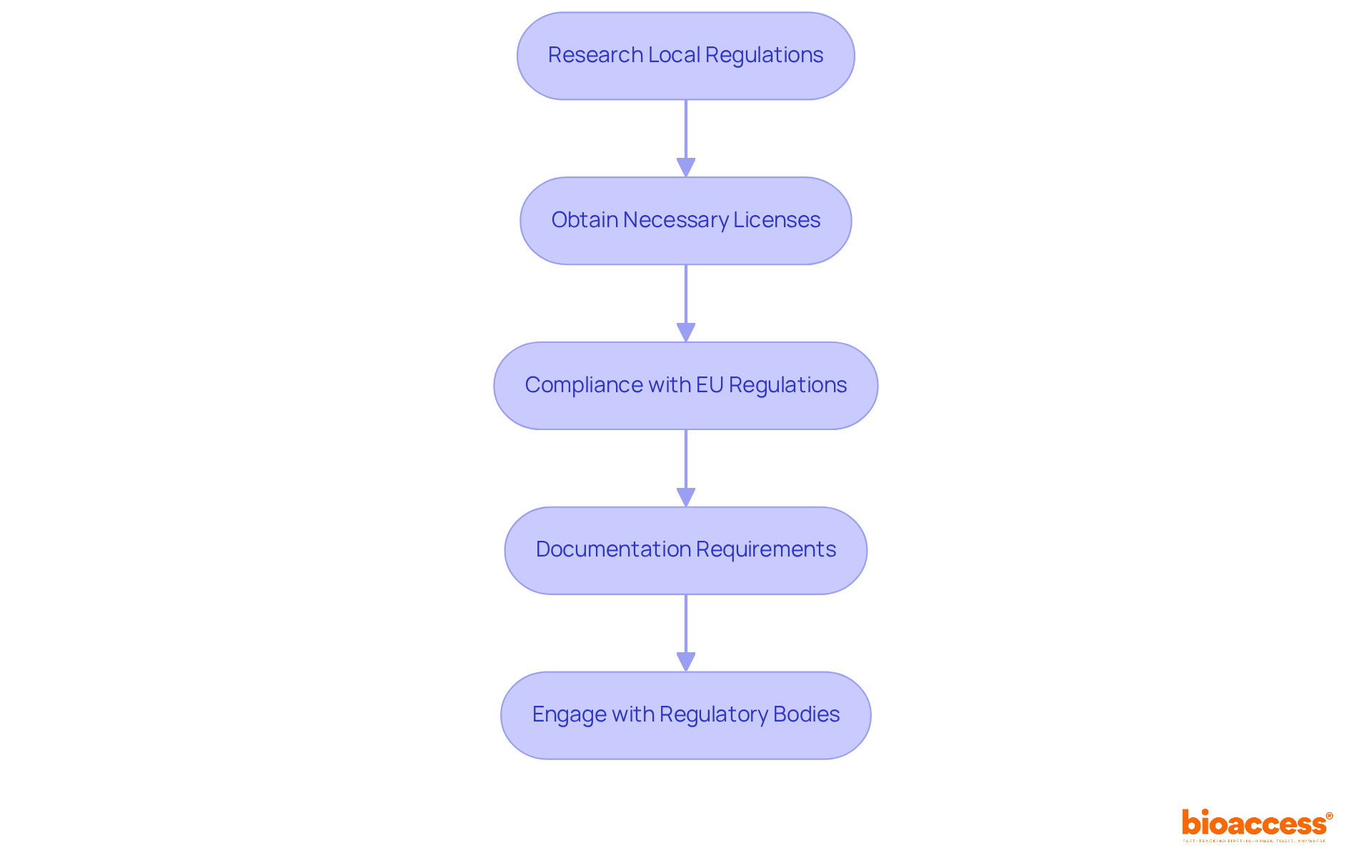
To effectively navigate the regulatory landscape for drug import and distribution in Croatian clinical trials, follow these essential steps:
Research Local Regulations: Begin by reviewing the Act on Medicinal Products and the Ordinance on Clinical Trials. These documents outline the legal prerequisites for conducting clinical studies and importing medications in Croatia, providing a foundational understanding of the regulatory framework.
Obtain Necessary Licenses: Secure all required licenses for importing Investigational Medicinal Products (IMPs). This includes obtaining an import license from HALMED, which typically requires up to 15 working days for processing. Understanding this timeline is crucial for organizing your activities efficiently.
Compliance with EU Regulations: Familiarize yourself with the EU Clinical Trials Regulation (EU CTR) No. 536/2014, which governs proceedings conducted in Croatia. This regulation standardizes clinical research processes across EU member states, making adherence essential for successful execution.
Documentation Requirements: Prepare all necessary documentation, including ethical approvals, clinical study applications, and safety reports. Following local guidelines for document submission is vital to avoid delays and ensure a smooth approval process.
Engage with Regulatory Bodies: Maintain open communication with HALMED and other relevant regulatory authorities to clarify compliance uncertainties and stay informed about any regulatory changes. This proactive approach can facilitate smoother interactions and enhance your understanding of the regulatory environment.
By following these steps, you will ensure that your clinical studies comply with Croatian regulations, which is essential for drug import and distribution tracking in Croatian trials and significantly reducing the risk of legal complications.
To establish an effective drug tracking system for clinical trials in Croatia, follow these essential steps:
Select Appropriate Technology: Choose a monitoring technology that suits your trial's needs. Options include barcode scanning, RFID, or IoT-based systems that offer real-time monitoring capabilities.
Develop Standard Operating Procedures (SOPs): Create SOPs that outline the processes for pharmaceutical importation, including obtaining necessary import permits, storage, distribution, and monitoring. Ensure that these procedures comply with regulatory requirements and best practices, reflecting the thorough compliance reviews offered by Bioaccess in relation to drug import and distribution tracking in Croatian trials.
Implement a Centralized Database: Utilize a centralized database to record all monitoring data. This database should contain information on medication batches, expiration dates, and distribution logs to enable easy access and reporting, aligning with Bioaccess's project management and monitoring services.
Train Staff on the Platform: Ensure that all team members involved in drug handling and monitoring are trained on the new platform. This training should cover the use of technology, SOPs, and compliance requirements, which are critical components of Bioaccess's trial setup and start-up processes.
Conduct Regular Audits: Arrange frequent evaluations of the monitoring framework to ensure adherence and pinpoint areas for enhancement. This will help preserve the integrity of the monitoring process and ensure that any discrepancies are addressed promptly, in line with Bioaccess's commitment to comprehensive reporting on study status and adverse events.
By implementing these steps, including the necessary considerations for import permits and nationalization of investigational devices, you will create a robust medication monitoring framework that enhances the efficiency and compliance of your clinical trials, particularly through drug import and distribution tracking in Croatian trials, supported by the extensive services provided by Bioaccess.
![]()
To ensure your team is well-prepared to effectively use the drug tracking system, follow these essential training steps:
Develop a Comprehensive Training Plan: Clearly outline objectives, content, and timelines for training sessions. Incorporate both theoretical and practical components to enhance understanding and retention.
Utilize Diverse Training Formats: Engage team members by incorporating various training formats, such as workshops, online modules, and hands-on demonstrations. This approach caters to different learning styles and fosters a more engaging learning environment.
Provide Access to Resources: Ensure that team members have access to essential training materials, user manuals, and FAQs related to the monitoring platform. This ongoing support reinforces learning and aids in knowledge retention.
Conduct Simulation Exercises: Arrange simulations where team members can practice utilizing the monitoring framework in a controlled environment. This practical experience enhances confidence and skill before the platform becomes operational. Simulations have been shown to reduce noncompliance and enhance proficiency, making them a critical component of effective training.
Gather Feedback and Adjust Training: After each training session, collect feedback from participants to identify areas for improvement. This iterative process allows for the refinement of future training sessions and effectively addresses any knowledge gaps.
By applying these strategies, you will equip your team with the essential abilities to effectively use the drug import and distribution tracking in Croatian trials, ensuring seamless operations during clinical trials. As Harvey S. Firestone noted, "the growth and development of people is the highest calling of leadership," making effective training a vital investment in your team's success.
![]()
To effectively monitor and evaluate the performance of your drug tracking system, consider implementing the following strategies:
Establish Key Performance Indicators (KPIs): Define specific KPIs to evaluate the efficiency of the monitoring framework. Important metrics may include the accuracy of monitoring data, the time taken for drug delivery, compliance rates, and protocol adherence, which are crucial for maintaining trial integrity.
Conduct Regular Reviews: Schedule consistent evaluations of the monitoring mechanism's performance against the established KPIs. This practice aids in recognizing trends, opportunities for enhancement, and compliance challenges that may occur, ensuring that the framework operates efficiently.
Utilize Feedback Mechanisms: Establish feedback channels for team members to report challenges or inconsistencies encountered while using the monitoring tool. Gathering insights from users can highlight potential areas for enhancement and foster a culture of continuous improvement.
Adjust Processes as Needed: Be prepared to modify processes, standard operating procedures (SOPs), or training programs based on performance evaluations and feedback. This flexibility is crucial for enhancing the efficiency of the monitoring framework and ensuring adherence to regulatory standards, including securing required ethics committee approvals before study commencement.
Document Findings and Actions: Keep comprehensive records of performance assessments, discoveries, and any measures implemented to enhance the monitoring framework. This documentation is vital for regulatory compliance and will be invaluable during future audits.
By implementing these strategies, you will ensure that your drug tracking system remains effective, compliant, and capable of supporting the successful execution of clinical trials.
![]()
Mastering drug import and distribution tracking is essential for the success of clinical trials in Croatia. Understanding the regulatory landscape and implementing effective tracking systems not only ensures compliance but also maintains the integrity of investigational medicinal products throughout the trial process. This article serves as a comprehensive guide to establishing a robust drug tracking framework, highlighting the critical importance of regulatory compliance, technology integration, and team training.
Key insights include:
Moreover, developing standard operating procedures and conducting thorough training for staff are vital components that contribute to the seamless operation of drug tracking systems. Regular performance evaluations and feedback mechanisms further enhance the effectiveness of these systems, ensuring that any issues are promptly addressed.
In conclusion, the significance of effective drug tracking in clinical trials cannot be overstated. It safeguards the integrity of the trial process and fosters trust among stakeholders, including regulatory bodies and participants. By prioritizing the establishment of a comprehensive tracking system and committing to continuous improvement, clinical trial teams can enhance their operational efficiency and uphold the highest standards of compliance in drug import and distribution tracking within Croatia.
What is drug importation in the context of Croatian clinical trials?
Drug importation refers to the legal process of bringing investigational medicinal products (IMPs) into Croatia, which requires necessary documentation such as import licenses and compliance with local regulations.
Why is drug import and distribution tracking important in clinical trials?
It ensures the systematic monitoring of medication movement from importation to clinical trial sites, maintaining the integrity of medications and ensuring they are delivered safely and efficiently.
What are the key regulatory requirements for drug importation in Croatia?
Key regulatory requirements include understanding the Act on Medicinal Products, obtaining necessary licenses from HALMED, and complying with the EU Clinical Trials Regulation (EU CTR) No. 536/2014.
How long does it typically take to process an import license from HALMED?
It typically requires up to 15 working days for processing an import license from HALMED.
What documentation is necessary for conducting clinical trials in Croatia?
Necessary documentation includes ethical approvals, clinical study applications, and safety reports, which must be prepared according to local guidelines to avoid delays.
How can technology enhance drug import and distribution tracking?
Utilizing contemporary monitoring technologies such as RFID and IoT can enhance the efficiency of medication oversight and provide real-time visibility of item status throughout the supply chain.
What steps should be taken to ensure compliance with Croatian regulations?
Steps include researching local regulations, obtaining necessary licenses, preparing documentation, and engaging with regulatory bodies like HALMED to clarify compliance uncertainties.
Why is it important to maintain communication with regulatory authorities?
Maintaining open communication with regulatory authorities helps clarify compliance uncertainties and keeps you informed about any regulatory changes, facilitating smoother interactions and better understanding of the regulatory environment.