


Navigating the complex landscape of ethics compliance in Serbian clinical trials demands a comprehensive understanding of regulatory frameworks and best practices. With the number of ongoing studies in Serbia on the rise, the necessity for meticulous ethics compliance audits becomes critical. These audits are essential not only for safeguarding participant welfare but also for upholding the integrity of research. However, as regulations evolve and local requirements grow more intricate, researchers must ask themselves:
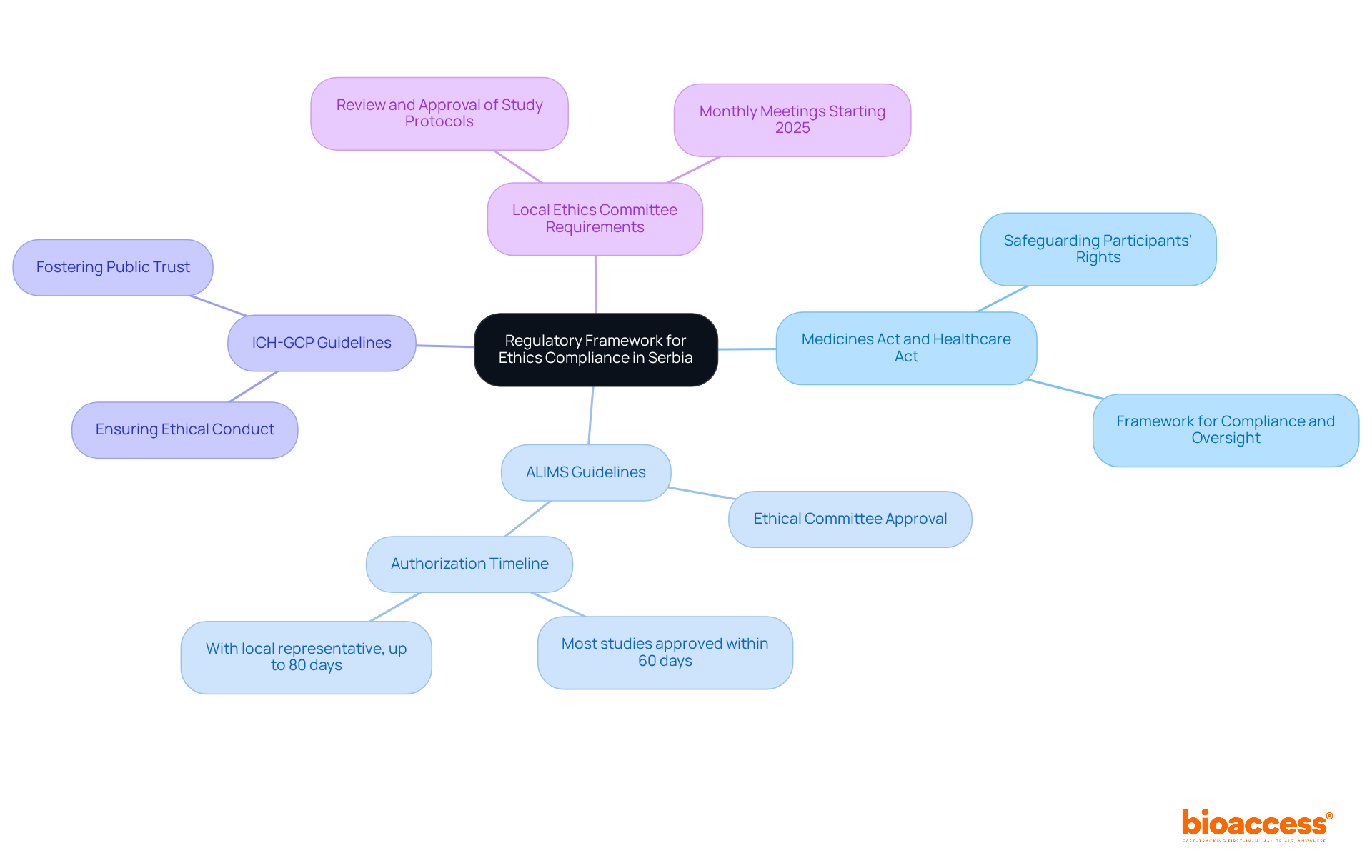
To effectively conduct an ethics compliance audit in Serbia, understanding key regulations is essential:
Medicines Act and Healthcare Act: These foundational laws govern the ethical conduct of medical studies, safeguarding participants' rights and welfare. They establish a framework for compliance and oversight in medical research.
ALIMS Guidelines: The Agency for Medicines and Medical Devices of Serbia (ALIMS) sets specific criteria for research studies, emphasizing the necessity of ethical committee approval. Recent updates show that most studies obtain authorization within 60 days, and with a local representative, this can extend to 80 days. This reflects a commitment to efficient regulatory processes.
ICH-GCP Guidelines: The International Council for Harmonisation's Good Clinical Practice (ICH-GCP) guidelines are vital for ensuring studies are conducted ethically and scientifically valid. Adhering to these guidelines fosters public trust in medical research and enhances the integrity of the data collected.
Local Ethics Committee (LEC) Requirements: Each research institution in Serbia has its own Local Ethics Committee (LEC) tasked with reviewing and approving study protocols. These committees ensure compliance with both national and international ethical standards, meeting monthly to evaluate applications starting in 2025 as part of a broader initiative to streamline the approval process.
By familiarizing yourself with these frameworks, including the fact that Serbia currently hosts 322 ongoing clinical studies, you can navigate the complexities of ethics compliance audits for Serbian clinical trials more effectively. This ensures that participant welfare is prioritized and that research integrity is maintained.
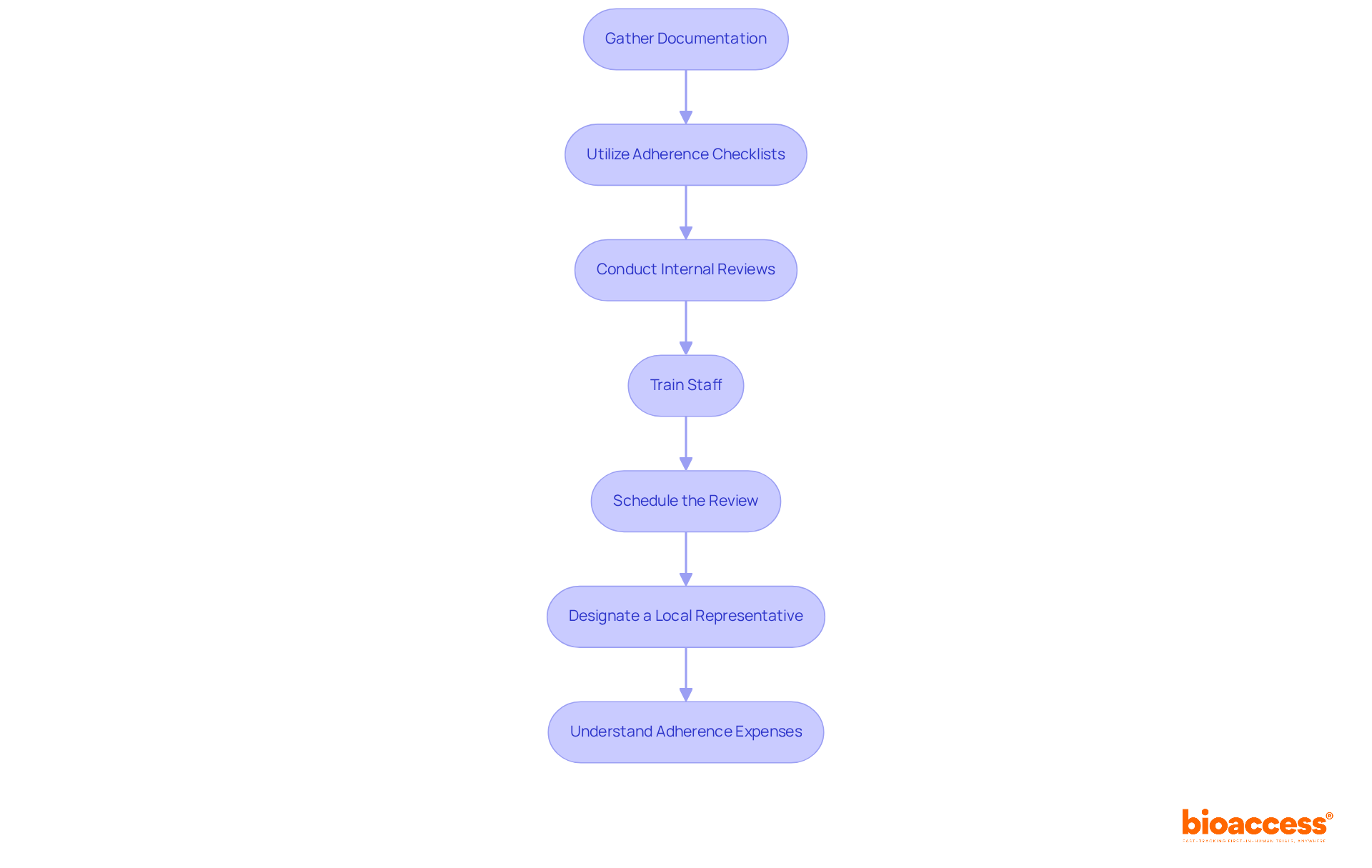
To effectively prepare for the ethics compliance audit, follow these essential steps:
Gather Documentation: Compile all pertinent documents, including trial protocols, informed consent forms, and ethics committee approvals. This step is vital, as ethics compliance audits for Serbian clinical trials necessitate thorough documentation as a key element in successful evaluations.
Utilize adherence checklists that align with Serbian regulations and ICH-GCP guidelines during ethics compliance audits for Serbian clinical trials. This ensures that all necessary aspects are addressed, minimizing the risk of oversight.
Conduct Internal Reviews: Implement internal reviews to identify discrepancies or areas of non-compliance prior to the official evaluation. This proactive approach can significantly enhance the likelihood of a favorable outcome in ethics compliance audits for Serbian clinical trials. Furthermore, performing practice audits can assist in recognizing weaknesses and enhancing preparation, demonstrating a commitment to adherence.
Train Staff: Ensure that all team members involved in the trial are well-trained on regulatory requirements and understand their roles in upholding ethical standards. Effective training fosters a culture of compliance and accountability, particularly in the context of ethics compliance audits for Serbian clinical trials.
Schedule the Review: Coordinate with the ethics committee and relevant stakeholders to arrange the review, ensuring that all parties are prepared and available. Timely scheduling is vital for a smooth audit process.
Designate a Local Representative: For overseas sponsors, designating a Local Representative is crucial for legal responsibility and adherence to Serbian research regulations. This representative will assist in navigating the regulatory landscape and ensure that ethics compliance audits for Serbian clinical trials meet local requirements.
Understand Adherence Expenses: Be aware that the average cost of non-adherence is 2.71 times higher than that of adherence. This statistic underscores the importance of thorough documentation and adherence to ethical standards.
By diligently following these steps, you establish a robust framework for a successful ethics verification, ultimately enhancing the integrity and credibility of your clinical trial.
When conducting ethics compliance audits for Serbian clinical trials, it’s crucial to adhere to best practices that ensure a thorough evaluation.
By applying these best practices, you can ensure that ethics compliance audits for Serbian clinical trials are thorough and effective, ultimately enhancing the integrity of clinical research.
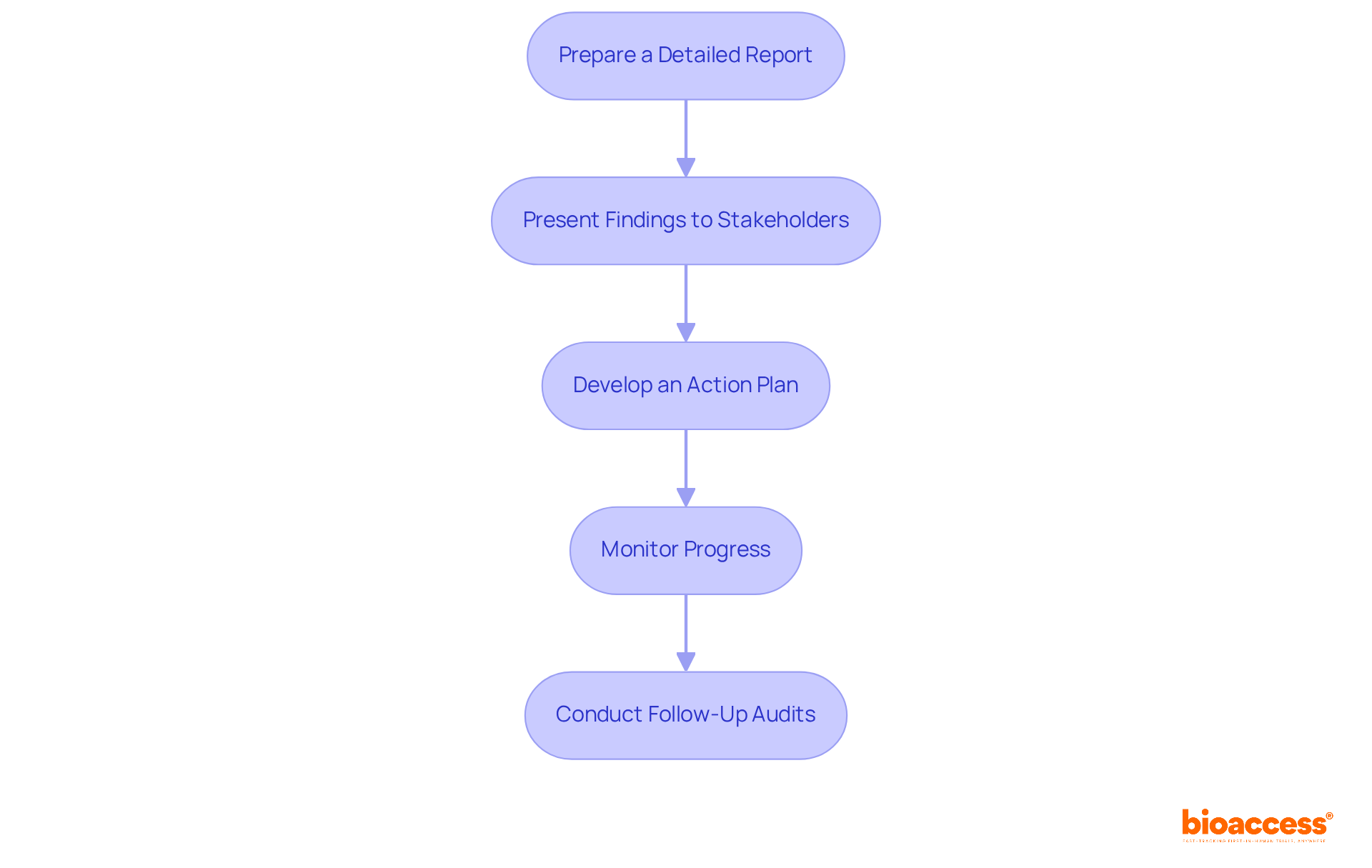
After completing the ethics compliance audits for Serbian clinical trials, it’s crucial to effectively report findings and implement improvements. Here’s how:
Prepare a Detailed Report: Summarize the examination results, clearly distinguishing between areas of adherence and non-adherence. Include actionable recommendations for improvement to guide stakeholders, especially in light of the 2025 regulatory updates that impact compliance processes.
Present Findings to Stakeholders: Share the report with key stakeholders, including the ethics committee and research sponsors, to promote transparency and accountability in the audit process. Highlight the significance of public registration of research studies and prompt reporting of outcomes to build trust.
Develop an Action Plan: Formulate a detailed action plan addressing identified non-compliance issues. This plan should assign specific responsibilities and establish timelines for implementation, ensuring clarity in accountability. Consider the need for sponsors and pharmaceutical companies to update regulatory processes to align with the FDAAA 801 Final Rule and international standards. Bioaccess provides extensive clinical trial management services, including feasibility studies, site selection, regulatory reviews, trial setup, import permits, project management, and reporting, which can assist this process.
Monitor Progress: Implement a robust system for tracking the progress of the action plan. Regular monitoring will help ensure that improvements are effectively made and maintained over time, especially given that penalties for non-compliance can now reach $15,000 per day.
Conduct Follow-Up Audits: Schedule follow-up audits to evaluate the effectiveness of the changes implemented. This ongoing evaluation is essential for ensuring sustained adherence to ethical standards and adapting to the evolving regulatory landscape.
By systematically reporting findings and executing improvements, you can significantly enhance the ethical conduct of clinical trials and implement ethics compliance audits for Serbian clinical trials, thereby cultivating a culture of compliance within your organization.
Understanding and mastering ethics compliance audits for Serbian clinical trials is not just important; it’s essential for ensuring the integrity and ethical conduct of medical research. Familiarizing oneself with the regulatory frameworks, preparing meticulously, and adhering to best practices during audits are critical steps that stakeholders must take to safeguard participant welfare and maintain public trust in clinical trials.
Key steps, such as gathering essential documentation, conducting internal reviews, and training staff, create a robust foundation for successful audits. Engaging stakeholders, establishing clear objectives, and thoroughly documenting findings are vital components of the auditing process. These measures not only enhance compliance but also foster a culture of accountability and transparency within research organizations.
Ultimately, the significance of ethics compliance in clinical trials cannot be overstated. As regulations evolve, particularly with the anticipated updates in 2025, it is imperative for researchers and sponsors to remain vigilant and proactive. By implementing the recommendations and action plans developed from audit findings, organizations can ensure sustained adherence to ethical standards. This commitment contributes to the advancement of trustworthy and responsible medical research in Serbia.
What are the key regulations for ethics compliance in Serbia?
The key regulations include the Medicines Act and Healthcare Act, ALIMS Guidelines, ICH-GCP Guidelines, and Local Ethics Committee (LEC) Requirements.
What do the Medicines Act and Healthcare Act govern?
These laws govern the ethical conduct of medical studies, safeguarding participants' rights and welfare, and establishing a framework for compliance and oversight in medical research.
What role does the Agency for Medicines and Medical Devices of Serbia (ALIMS) play in research studies?
ALIMS sets specific criteria for research studies, emphasizing the necessity of ethical committee approval and has updated processes to ensure most studies obtain authorization within 60 days.
How do ICH-GCP Guidelines contribute to medical research?
ICH-GCP Guidelines ensure that studies are conducted ethically and scientifically valid, fostering public trust in medical research and enhancing the integrity of the data collected.
What is the function of Local Ethics Committees (LEC) in Serbia?
Each research institution has its own LEC responsible for reviewing and approving study protocols to ensure compliance with national and international ethical standards.
How often do Local Ethics Committees meet to evaluate applications?
Starting in 2025, Local Ethics Committees will meet monthly to evaluate applications as part of an initiative to streamline the approval process.
How many clinical studies are currently ongoing in Serbia?
Serbia currently hosts 322 ongoing clinical studies.