


The article emphasizes the critical steps essential for achieving clinical success via the FDA Accelerated Approval program, designed to expedite access to promising treatments for serious conditions. It delineates vital processes such as:
Understanding these steps is imperative for researchers, as it enables them to navigate the complexities of drug development effectively, ultimately leading to improved health outcomes for patients.
The FDA Accelerated Approval program serves as a pivotal mechanism in the quest for timely access to groundbreaking therapies, particularly for patients confronting serious health challenges. By permitting drugs to gain approval based on early evidence of efficacy, this pathway not only enhances treatment availability but also emphasizes the critical role of rigorous clinical research.
However, navigating the complexities of this program raises essential questions:
This article explores the essential strategies and insights needed to master the FDA Accelerated Approval process, ensuring that innovative treatments reach those in need without unnecessary delays.
The FDA Accelerated Approval program is a crucial regulatory pathway that speeds up the authorization of medications and biologics designed to meet unmet medical needs, especially in severe conditions. This initiative allows for earlier endorsement based on preliminary evidence, such as surrogate endpoints that are reasonably likely to predict clinical benefit. In 2025, the FDA authorized 12 medications through this pathway, underscoring its ongoing importance within the healthcare landscape. The primary objective of this program is to facilitate prompt access to promising treatments, thereby improving health outcomes and swiftly addressing urgent medical needs compared to traditional authorization processes.
Recent analyses reveal that from 2013 to 2017, 63% of cancer medications granted expedited clearance transitioned to standard endorsement, highlighting the program's effectiveness. Notably, from 2006 to 2022, 69 expedited oncology therapies contributed approximately 263,000 life years for around 911,000 cancer patients, with projections suggesting this could rise to 382,000 life years by 2026.
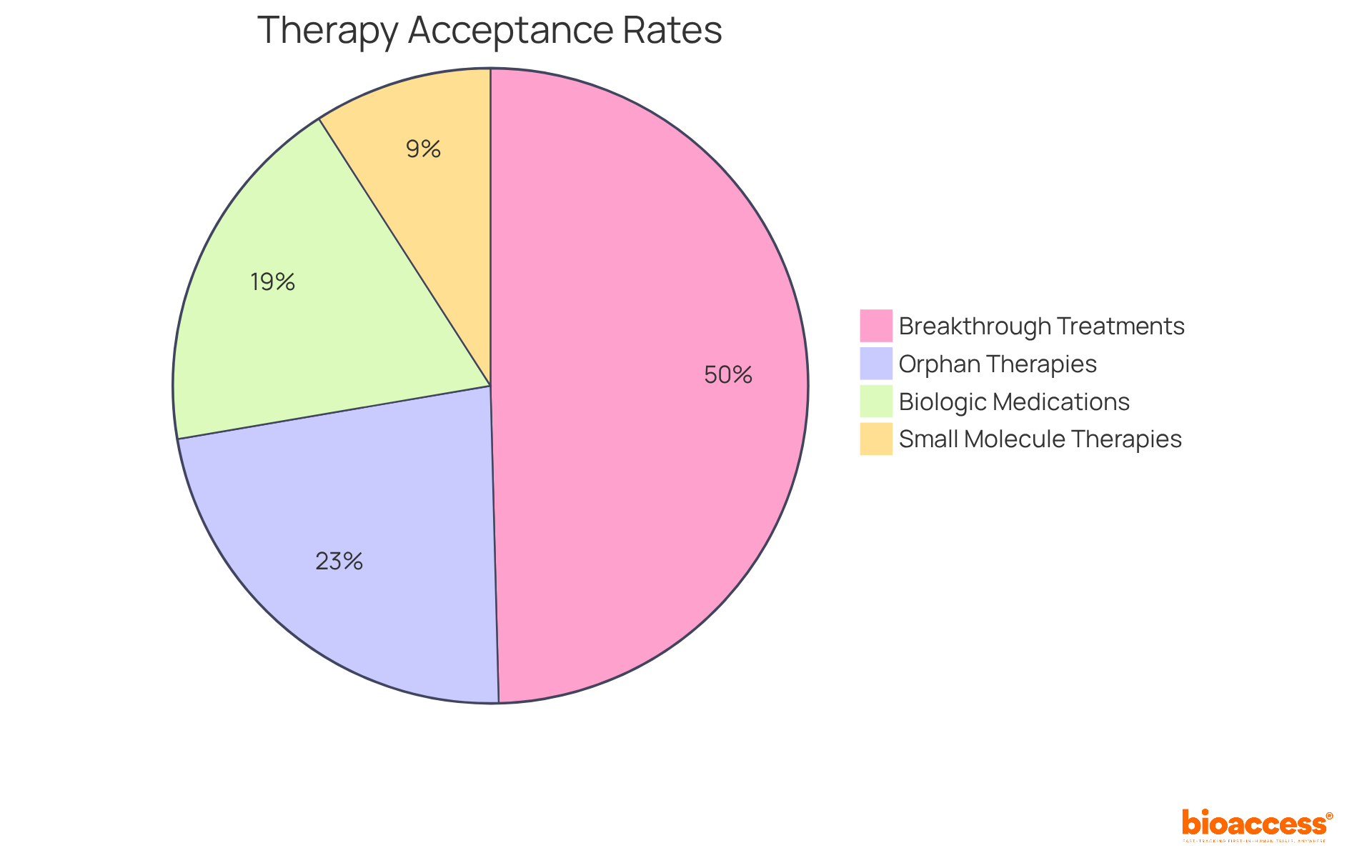
Successful real-world examples include orphan therapies, which boast a higher acceptance rate of 25%-30% due to regulatory incentives, and breakthrough-designated treatments, which have an acceptance rate of about 60%. Additionally, biologic medications show a validation rate of approximately 20%-25%, while small molecule therapies achieve a success rate of roughly 10%-12%. These statistics illustrate the program's significant role in advancing innovative treatments. As clinical researchers navigate the complexities of drug development, understanding the intricacies of the FDA accelerated approval program is vital for efficiently bringing effective therapies to market. As Peter J. Pitts, president of the Center for Medicine in the Public Interest, remarked, 'The accelerated approval pathway works, but it isn’t — nor is it intended to be — a flawless oracle of therapeutic success.
To qualify for FDA Accelerated Approval, a drug must meet specific criteria: it should address a serious condition and provide evidence showing that it affects a surrogate endpoint likely to predict clinical benefit. This process encompasses several essential steps:
In 2025, the FDA has observed a notable increase in the number of IND applications submitted for FDA accelerated approval, reflecting the growing interest in expediting access to promising therapies. Successful examples of IND applications leading to FDA accelerated approval highlight the pathway's potential to bring innovative treatments to market swiftly. However, ongoing updates to the FDA accelerated approval process emphasize the necessity for strong clinical research data and highlight the significance of post-marketing studies to ensure patient safety and treatment effectiveness. Understanding these criteria and processes is crucial for researchers navigating the FDA's regulatory landscape effectively.
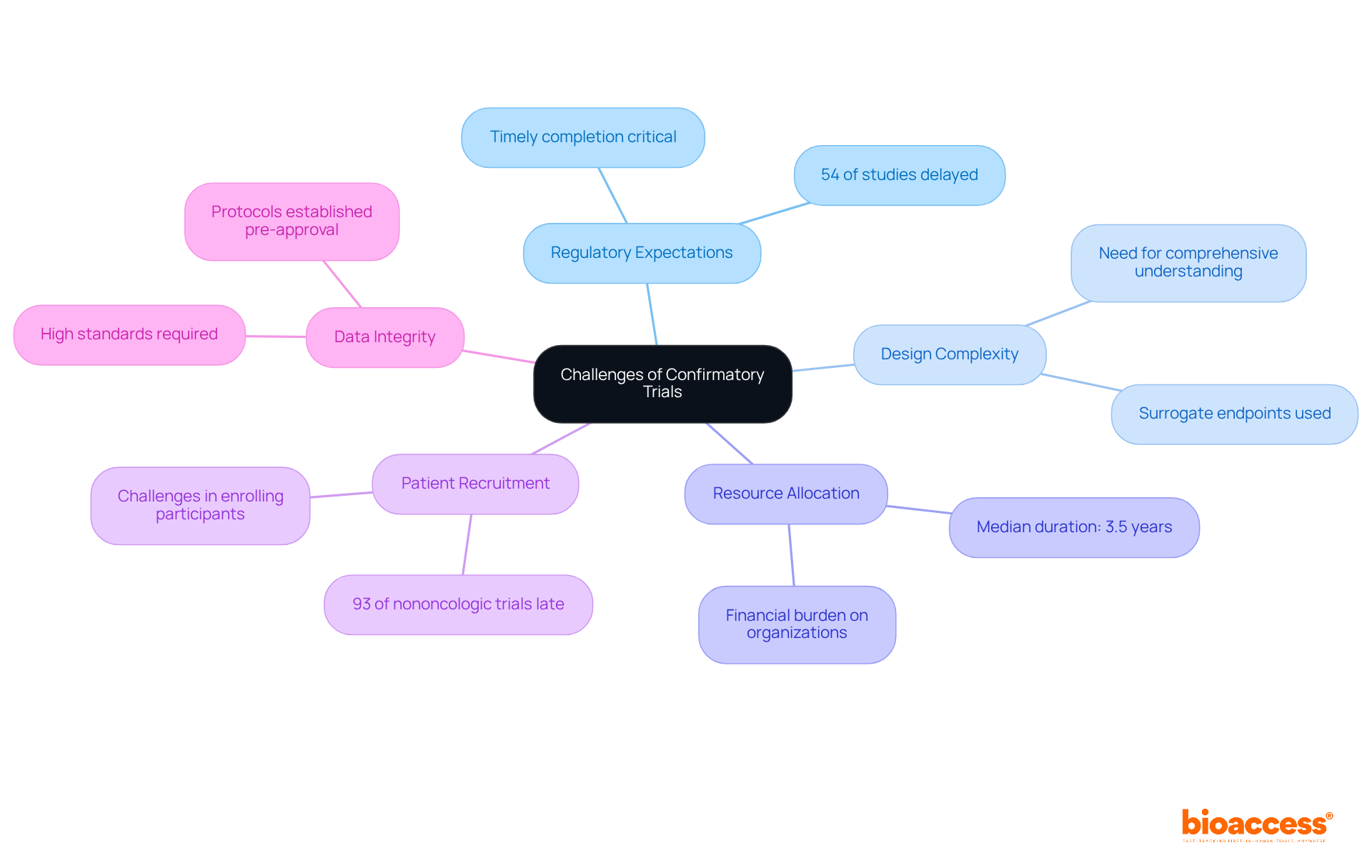
Confirmatory trials are essential in validating the clinical benefits of drugs granted Accelerated Approval, yet they present several implications and challenges that demand attention:
Regulatory Expectations: The FDA mandates that confirmatory studies be conducted swiftly, as delays can lead to the withdrawal of the drug from the market. This underscores the critical need for adherence to timelines. Notably, 54% of confirmatory studies experienced delays, highlighting the urgency and complexities involved in meeting these regulatory schedules. bioaccess® offers expertise in navigating these intricate regulatory demands, ensuring that studies are established effectively and in compliance with all necessary regulations.
Design Complexity: Developing confirmatory studies that convincingly demonstrate clinical benefit can be complex, especially when initial approvals relied on surrogate endpoints. This intricacy necessitates a comprehensive understanding of both the disease and the endpoints employed. bioaccess® provides extensive support in study design, including feasibility assessments and site selection, empowering organizations to create robust protocols that align with regulatory expectations.
Resource Allocation: Organizations must allocate sufficient resources and funding for these studies, which can be extensive and financially burdensome. The median duration for completing confirmatory studies is approximately 3.5 years, with a range from 1.8 to 5.8 years, emphasizing the variability in study completion times and the importance of strategic planning. bioaccess® assists in project management and monitoring, ensuring that resources are utilized efficiently throughout the testing process.
Patient Recruitment: Enrolling participants for confirmatory studies can pose challenges, particularly if the initial patient population was limited or if the condition is rare. This difficulty is exacerbated by the fact that tests for nononcologic indications have been late 93% of the time, indicating significant recruitment hurdles. bioaccess® offers feasibility studies and site selection services to enhance patient recruitment strategies, addressing these challenges directly.
Data Integrity: Upholding high standards of data integrity and compliance throughout the confirmatory study process is vital to ensuring the validity of results. The FDA's guidance emphasizes the importance of having protocols established prior to receiving FDA accelerated approval, which helps mitigate risks associated with data integrity. bioaccess® guarantees that all study documents meet country requirements, reinforcing the integrity of the data collected.
Navigating these challenges necessitates meticulous planning and a robust strategy to ensure that confirmatory trials not only fulfill regulatory expectations but also substantiate the clinical benefits of the drugs in question. Furthermore, the FDA's proactive measures regarding dangling authorizations, including the voluntary retraction of four authorizations between 2020 and 2021, illustrate the agency's commitment to effectively addressing these challenges.
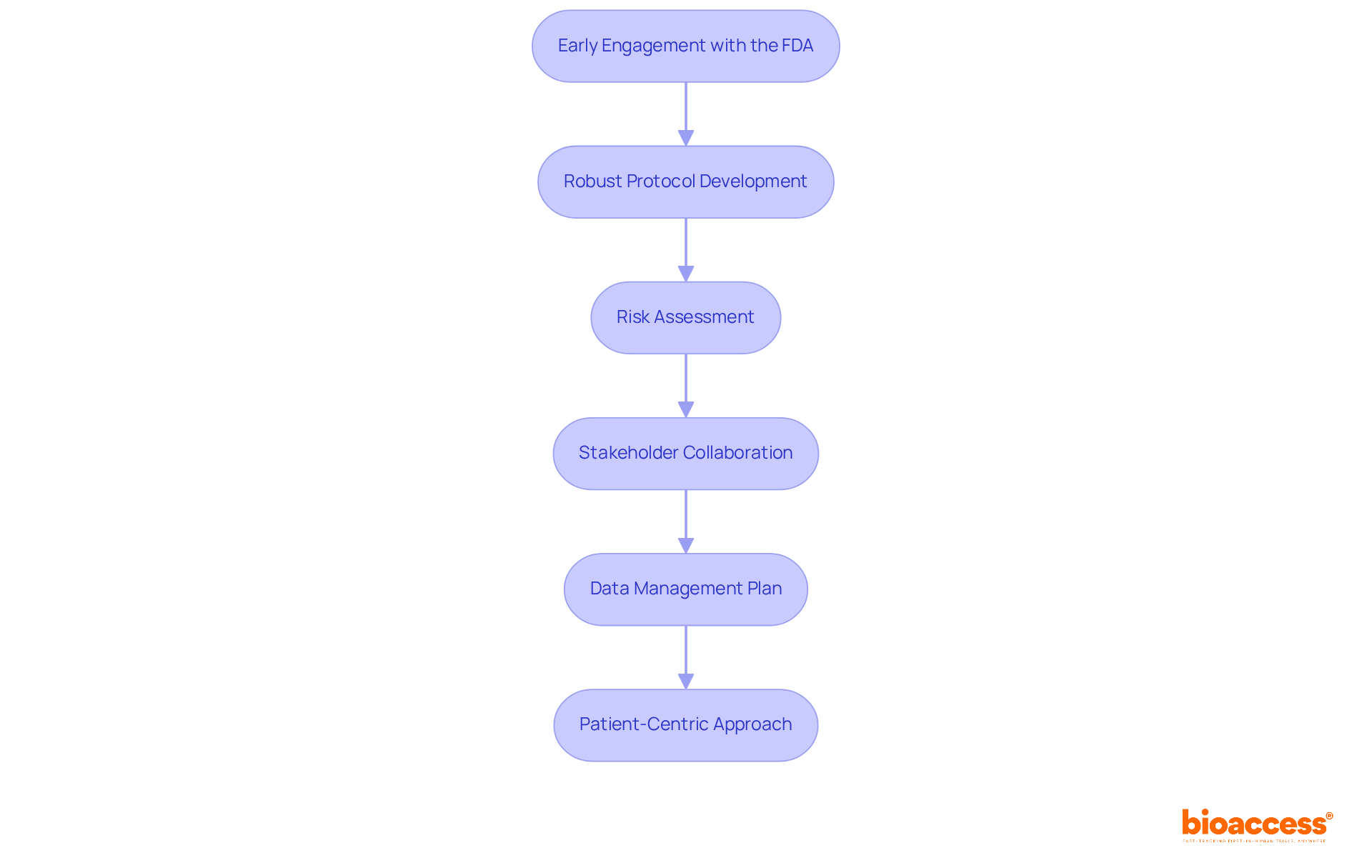
Effective clinical research planning for Accelerated Approval encompasses several strategic steps that are vital for success:
Early Engagement with the FDA: Initiating discussions with the FDA as early as one year prior to submitting an Investigational New Drug (IND) application is crucial. This proactive approach allows sponsors to clarify expectations and align on regulatory requirements, significantly reducing risks associated with the approval process. Engaging early can lead to a more efficient review process and better outcomes.
Robust Protocol Development: A comprehensive clinical study protocol is essential. It should clearly outline objectives, endpoints, and methodologies, ensuring that all aspects of the study are well-defined. Research indicates that experiments with strong protocols have greater success rates, particularly in managing the intricacies of regulatory examination.
Risk Assessment: Conducting a thorough risk assessment helps identify potential challenges early in the process. By developing mitigation strategies, sponsors can proactively address concerns, which is vital for maintaining momentum in the approval process.
Stakeholder Collaboration: Fostering collaboration among all stakeholders—including investigators, sponsors, and regulatory bodies—ensures a unified approach. This partnership is essential for tackling any problems that may arise during the evaluation and can enhance the overall quality of the submission.
Data Management Plan: Developing a robust data management strategy is essential for maintaining data integrity and compliance throughout the study. A well-structured plan supports the collection and analysis of data, which is critical for demonstrating the efficacy and safety of the treatment.
Patient-Centric Approach: Crafting studies with an emphasis on patient needs and experiences can significantly improve recruitment and retention rates. A patient-focused approach not only enhances study outcomes but also aligns with regulatory expectations for demonstrating clinical benefit. bioaccess® offers accelerated patient recruitment and site activation services, ensuring that trials are efficiently staffed and compliant with regulatory standards. Their expertise extends to managing various types of studies, including Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies, specifically in Latin America.
By implementing these strategies, researchers can significantly enhance their likelihood of achieving FDA accelerated approval, ultimately facilitating the successful introduction of innovative therapies to the market.
The FDA Accelerated Approval program serves as a vital mechanism in the healthcare system, enabling faster access to promising treatments for serious conditions. By prioritizing early authorization based on preliminary evidence, this initiative not only addresses unmet medical needs but also significantly enhances patient outcomes. Understanding the nuances of this program is essential for clinical researchers aiming to navigate the complexities of drug development effectively.
Key insights into the criteria and processes for achieving FDA Accelerated Approval have been discussed, including:
The challenges faced during these trials—such as regulatory expectations and patient recruitment issues—highlight the intricacies involved in ensuring that approved therapies deliver on their promised benefits. Furthermore, strategic planning and stakeholder collaboration emerge as critical components in successfully navigating the approval process.
Ultimately, the significance of the FDA Accelerated Approval program cannot be overstated. It represents a proactive approach in the fight against serious health conditions, urging researchers and stakeholders to embrace best practices in clinical research and regulatory compliance. By fostering a commitment to rigorous study design and patient-centric methodologies, the potential for innovative therapies to reach those in need can be greatly enhanced. Engaging with the FDA early and effectively will not only streamline the approval process but also contribute to a future where groundbreaking treatments become accessible in a timely manner.
What is the FDA Accelerated Approval program?
The FDA Accelerated Approval program is a regulatory pathway that expedites the authorization of medications and biologics designed to address unmet medical needs, particularly in severe conditions. It allows for earlier approval based on preliminary evidence, such as surrogate endpoints that can predict clinical benefit.
What is the primary purpose of the FDA Accelerated Approval program?
The primary purpose of the program is to facilitate prompt access to promising treatments, improving health outcomes and addressing urgent medical needs more rapidly than traditional authorization processes.
How many medications were authorized through the FDA Accelerated Approval pathway in 2025?
In 2025, the FDA authorized 12 medications through the Accelerated Approval pathway.
What percentage of cancer medications granted expedited clearance transitioned to standard endorsement from 2013 to 2017?
From 2013 to 2017, 63% of cancer medications granted expedited clearance transitioned to standard endorsement.
How many life years did expedited oncology therapies contribute from 2006 to 2022?
From 2006 to 2022, 69 expedited oncology therapies contributed approximately 263,000 life years for around 911,000 cancer patients.
What are the projected contributions of expedited oncology therapies to life years by 2026?
Projections suggest that expedited oncology therapies could contribute to approximately 382,000 life years by 2026.
What are the acceptance rates for orphan therapies and breakthrough-designated treatments under the FDA Accelerated Approval program?
Orphan therapies have an acceptance rate of 25%-30%, while breakthrough-designated treatments have an acceptance rate of about 60%.
What are the validation and success rates for biologic medications and small molecule therapies?
Biologic medications have a validation rate of approximately 20%-25%, while small molecule therapies achieve a success rate of roughly 10%-12%.
Why is understanding the FDA Accelerated Approval program important for clinical researchers?
Understanding the intricacies of the FDA Accelerated Approval program is vital for clinical researchers to efficiently bring effective therapies to market amidst the complexities of drug development.