


FDA Class 1 medical device regulations play a crucial role in facilitating swift market entry. This is primarily achieved through the exemption from the premarket notification procedure (510(k)), which allows manufacturers to bypass extensive equivalence demonstrations. While these devices must adhere to general controls, including proper labeling and Good Manufacturing Practices, it is noteworthy that nearly 74% are exempt from additional premarket requirements. This significantly streamlines the approval process, enabling rapid commercialization and addressing key challenges in the Medtech landscape.
Navigating the landscape of FDA Class 1 medical devices presents a unique opportunity for manufacturers aiming for swift market entry. With nearly half of all medical devices in the U.S. falling under this category, understanding the associated regulations and exemptions is crucial for ensuring compliance and successful product launches.
However, the complexities of maintaining quality standards while leveraging the benefits of expedited approval raise important questions:
Exploring these dynamics reveals strategies that can streamline the process and enhance the safety and efficacy of healthcare solutions.
FDA Class 1 medical devices are categorized as posing the least risk to patients and users, regulated by general controls to ensure their safety and effectiveness. Common examples include:
Notably, almost 50% of healthcare devices in the U.S. sector are categorized as FDA Class 1 medical devices, underscoring their prevalence and importance in patient care. A significant benefit of FDA Class 1 medical devices is that most are exempt from the premarket notification procedure, known as 510(k), which simplifies the route to entry. This exemption is crucial for manufacturers seeking rapid commercialization, as it reduces the regulatory burden and accelerates access to the market.
Understanding the nuances of the FDA Class 1 medical device classification is essential for manufacturers, as it directly impacts the regulatory requirements they must fulfill to ensure compliance and a successful product launch. Furthermore, manufacturers must maintain a Quality Management System (QMS) to comply with FDA regulations, and they must adhere to specific labeling requirements to ensure accurate representation of their products. Non-compliance with these regulations can lead to serious consequences, including Warning Letters or product seizures.
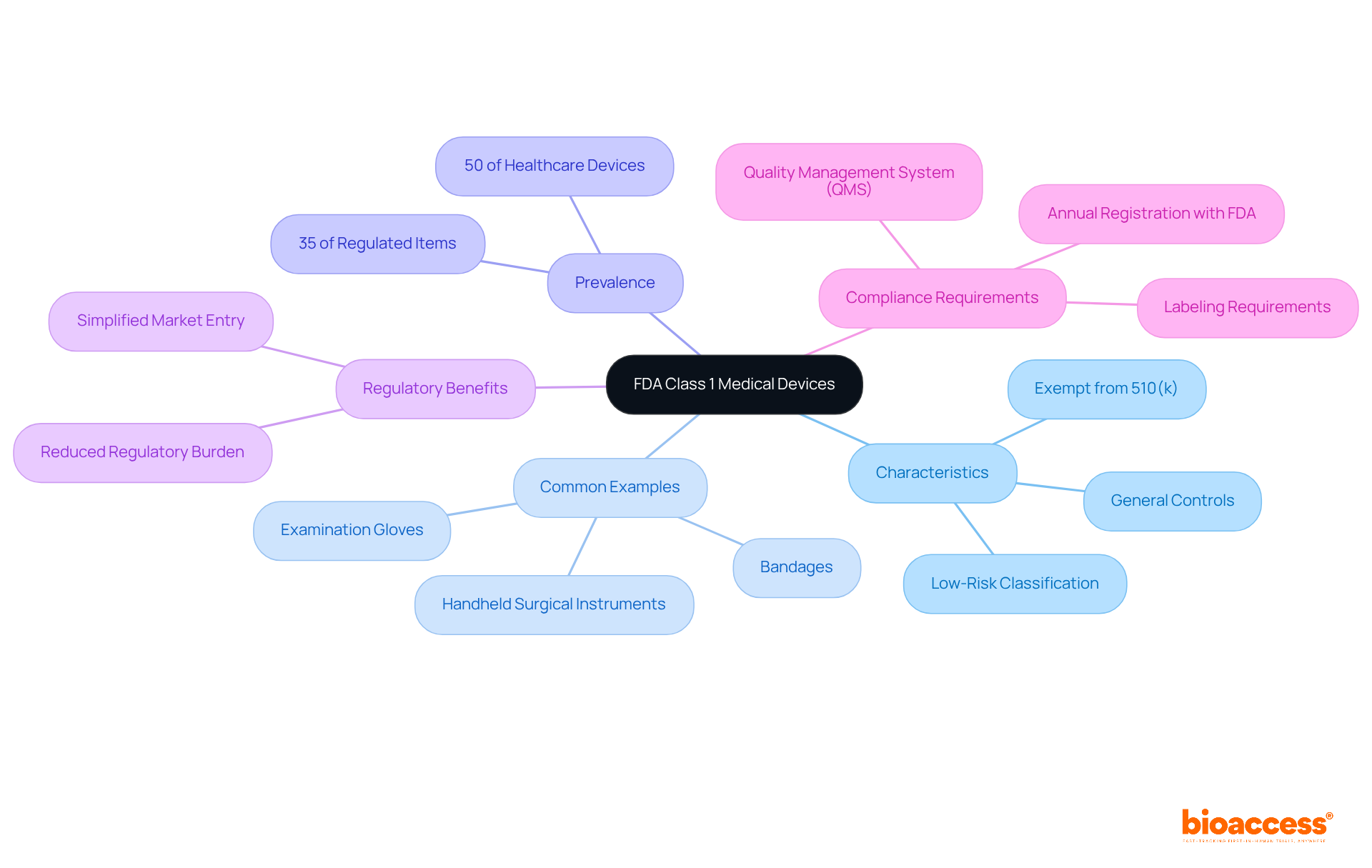
FDA class 1 medical devices predominantly enjoy exemptions from the 510(k) premarket notification requirements, allowing them to bypass the necessity of demonstrating substantial equivalence to a predicate instrument. However, it is essential to recognize that compliance with general controls is mandatory. This requirement includes:
Notably, certain FDA Class 1 medical devices may not qualify for these exemptions if their intended use poses significant risks to patient safety. Therefore, producers are strongly encouraged to consult the FDA's exemption list to verify their product's status and ensure compliance with all applicable regulations. This proactive approach not only facilitates quicker market entry but also underpins the delivery of safe and effective healthcare solutions.
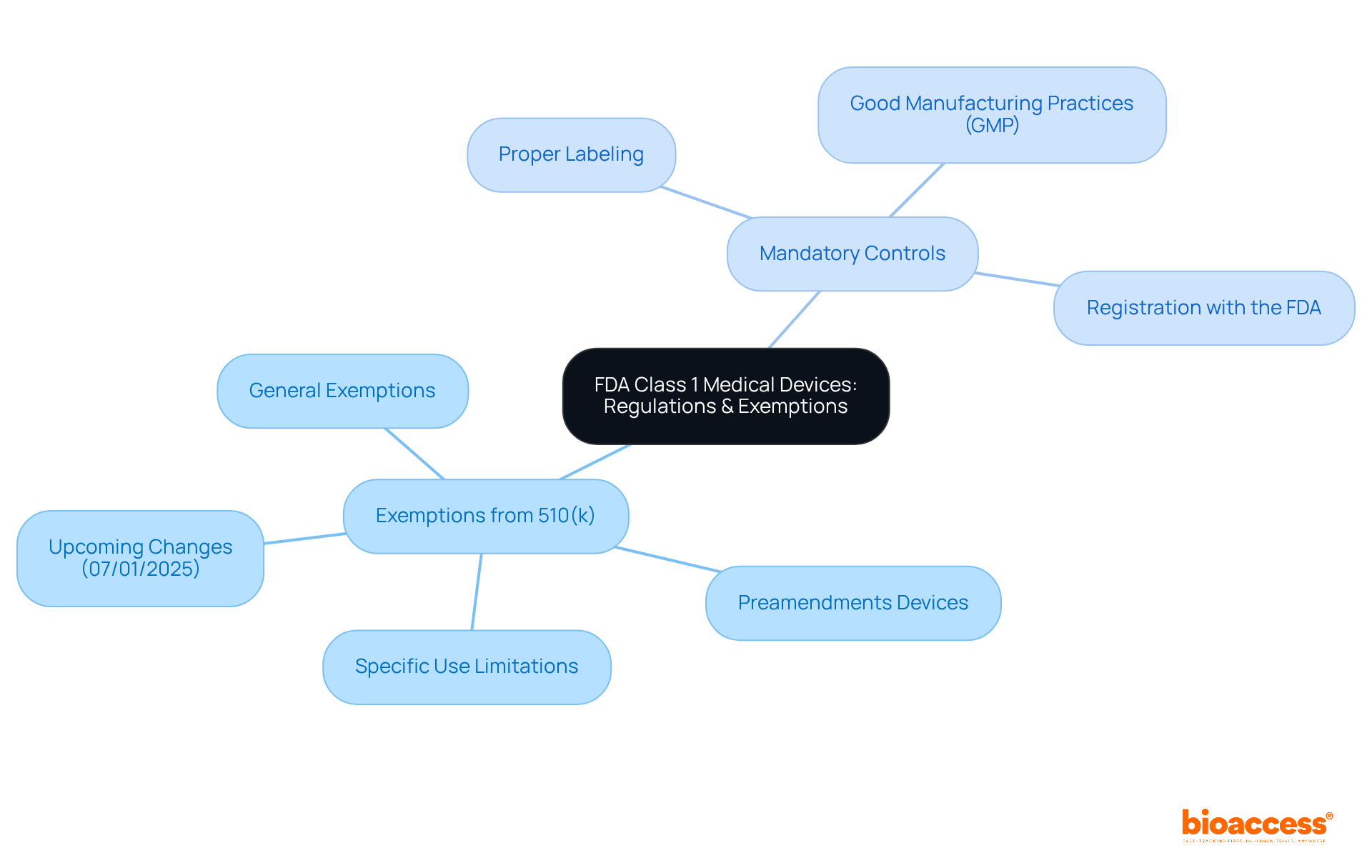
The approval procedure for an FDA Class 1 medical device is typically straightforward due to its low-risk categorization. Producers must register their establishment with the FDA and enumerate their products. Most Category 1 items are excluded from the 510(k) clearance route, significantly facilitating faster market entry.
While the schedule for entry can vary, many Category 1 products can be promoted within a few weeks to months after registration, provided they meet all regulatory criteria. The self-registration process can often be completed in about one week, enabling rapid access to the market.
Industry insights suggest that maintaining thorough documentation and proactive communication with the FDA can further expedite this process. Experts like Ana Criado, Director of Regulatory Affairs and a professor with extensive experience in biomedical engineering and health economics, emphasize the importance of staying informed about regulatory changes, particularly as the landscape evolves leading into 2025.
While the average duration for FDA approval under the traditional 510(k) pathway is approximately 177 days, it is crucial to note that this primarily pertains to Class 2 products, as most FDA Class 1 medical devices do not require this pathway. Furthermore, only 19% of products on the 510(k) pathway are approved within three months, highlighting the challenges faced in that approval process.
Engaging with regulatory affairs resources, including those provided by specialists like Ana Criado and Katherine Ruiz, can assist manufacturers in navigating complexities and minimizing delays, ensuring a more seamless route to entry.
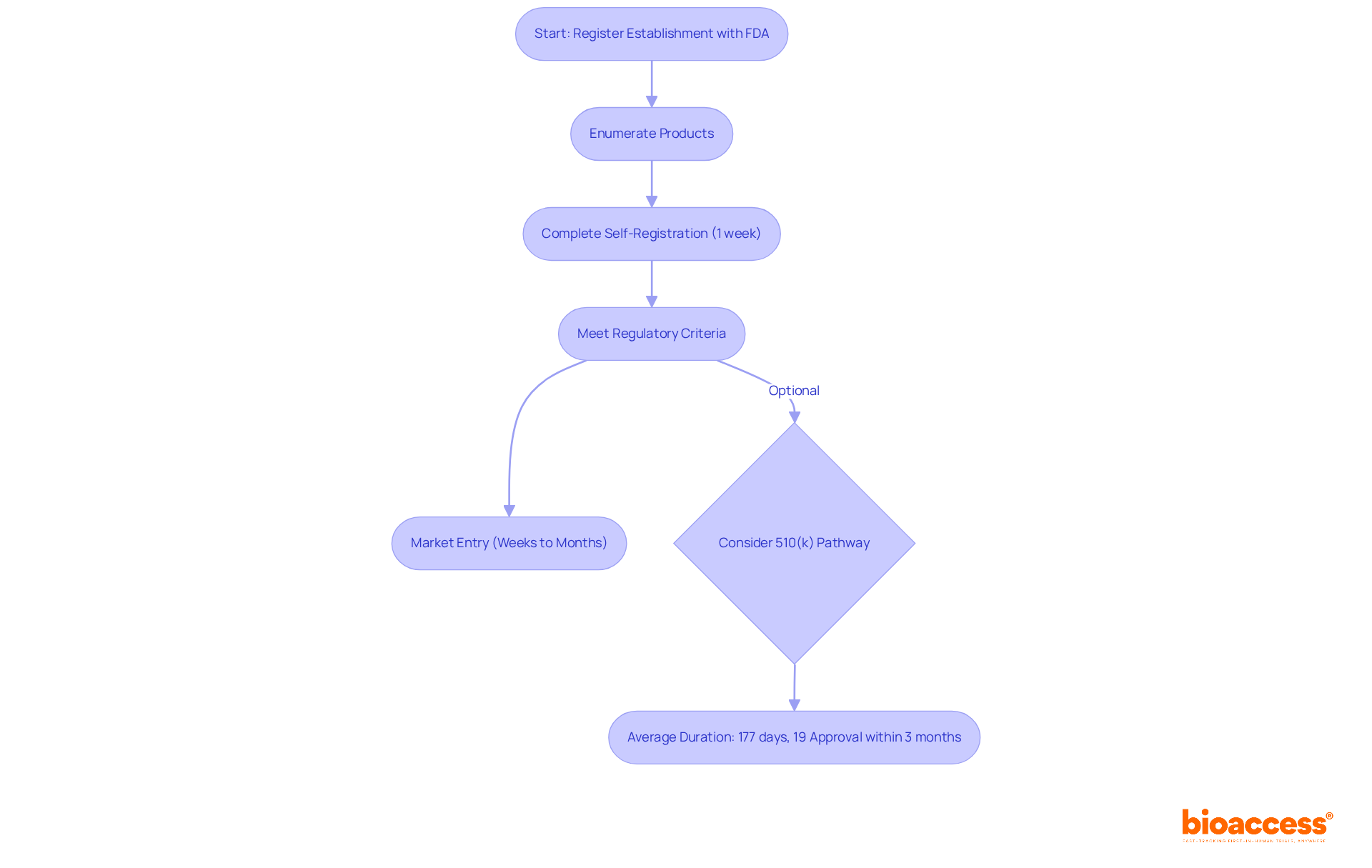
To achieve compliance with FDA regulations for FDA Class 1 medical devices, manufacturers must implement a comprehensive Quality Management System (QMS) that aligns with Good Manufacturing Practices (GMP) for these products. This necessitates ongoing training for staff on compliance requirements, detailed documentation of processes, and proactive risk management strategies.
Collaborating with industry experts, such as Ana Criado, Director of Regulatory Affairs and CEO of Mahu Pharma, and Katherine Ruiz, a specialist in Regulatory Affairs for Medical Devices and In Vitro Diagnostics in Colombia, can significantly aid manufacturers in navigating the complexities of compliance. Their vast expertise in biomedical engineering, health economics, and regulatory affairs offers invaluable insights for ensuring adherence to regulations.
By embracing these best practices, manufacturers not only secure regulatory compliance but also foster a culture of quality and safety within their organizations. Furthermore, approximately 74% of products categorized as FDA Class 1 medical devices are exempt from the premarket notification procedure, facilitating quicker market entry while still necessitating compliance with essential quality standards.
Examples of FDA Class 1 medical devices, such as latex gloves and surgical masks, highlight the types of items that must adhere to these regulations. This strategic approach not only enhances operational efficiency but also positions manufacturers advantageously within the competitive landscape of medical devices.
Navigating the landscape of FDA Class 1 medical devices is crucial for manufacturers aiming for swift market entry. These devices, characterized by their low-risk nature and exemption from extensive premarket notification procedures, represent a significant portion of the healthcare sector. Understanding the regulatory framework, including compliance with general controls and maintaining a robust Quality Management System, is essential for ensuring safety and effectiveness in patient care.
Throughout this article, key points have highlighted the importance of regulatory exemptions that facilitate quicker market access while emphasizing the necessity of adhering to established guidelines. From proper labeling to Good Manufacturing Practices, compliance is not merely a formality but a foundation for building trust in healthcare products. Engaging with experts and staying informed about evolving regulations will further streamline the approval process, enabling manufacturers to bring their innovations to market efficiently.
As the healthcare landscape continues to evolve, the significance of FDA Class 1 regulations cannot be overstated. Manufacturers are encouraged to proactively embrace compliance strategies and best practices to not only meet regulatory requirements but also enhance the quality and safety of their products. By doing so, they position themselves for success in a competitive market, ultimately contributing to improved patient outcomes and advancing the field of medical technology.
What are FDA Class 1 medical devices?
FDA Class 1 medical devices are categorized as posing the least risk to patients and users, regulated by general controls to ensure their safety and effectiveness.
Can you provide examples of FDA Class 1 medical devices?
Common examples of FDA Class 1 medical devices include bandages, examination gloves, and handheld surgical instruments.
What percentage of healthcare devices in the U.S. are FDA Class 1 medical devices?
Almost 50% of healthcare devices in the U.S. sector are categorized as FDA Class 1 medical devices.
What is a significant benefit of FDA Class 1 medical devices regarding market entry?
Most FDA Class 1 medical devices are exempt from the premarket notification procedure, known as 510(k), which simplifies the route to entry and reduces the regulatory burden for manufacturers.
Why is the exemption from the 510(k) procedure important for manufacturers?
The exemption is crucial for manufacturers seeking rapid commercialization, as it accelerates access to the market.
What must manufacturers maintain to comply with FDA regulations for Class 1 devices?
Manufacturers must maintain a Quality Management System (QMS) to comply with FDA regulations.
Are there specific labeling requirements for FDA Class 1 medical devices?
Yes, manufacturers must adhere to specific labeling requirements to ensure accurate representation of their products.
What are the consequences of non-compliance with FDA regulations for Class 1 devices?
Non-compliance can lead to serious consequences, including Warning Letters or product seizures.